Z991 FluorPen is a chlorophyll fluorescence measuring device for the lab and the field. This hand-held fluorometer uses high output LEDs to provide a saturating pulse for the measurement of Fv/Fm (QY) and other parameters. The Z991 FluorPen is an accurate, portable option to bulky fiber optic chlorophyll fluorometers, especially when the user wants to screen a large number of plants for specific fluorescence parameters.
Four different standard versions of FluorPen are available. All measure Ft, QY and kinetic responses such as OJIP, NPQ and Light Curve responses of QY, but have either a built in standard leaf clip (Z991) or are adapted to be used with detachable leaf clips (Z991-D). The Fluorpen can also be purchased with a build in PAR meter for measurements in 400-700nm range (Z991-LM or Z991-LM-D)
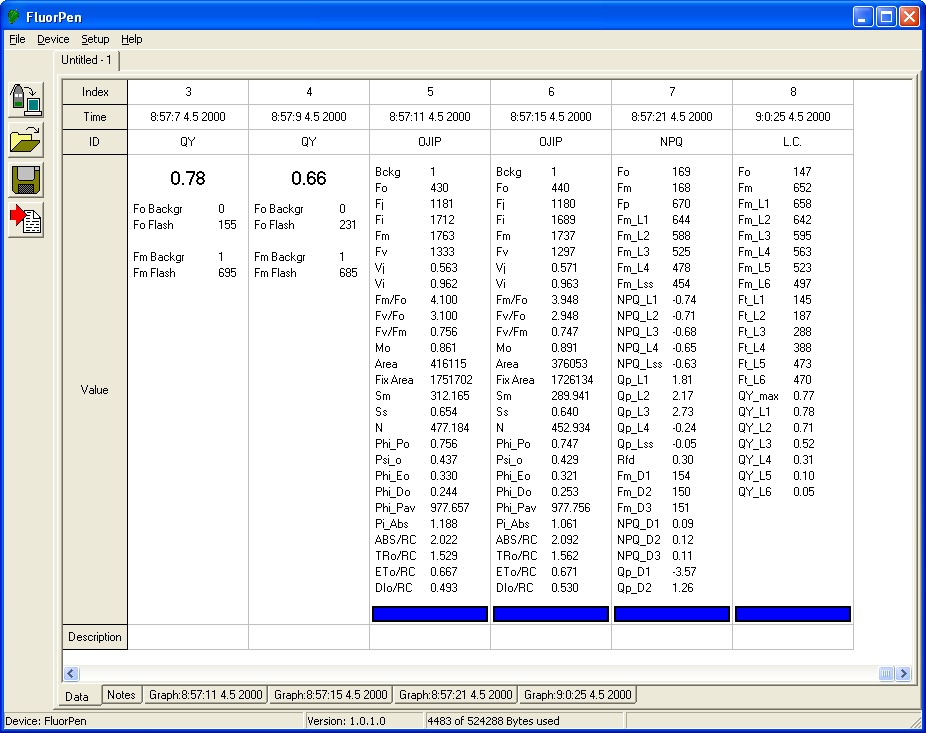
- Ft – continuous fluorescence yield in non-actinic light. Ft is equivalent to Fo if the leaf sample is dark-adapted.
- QY – Photosystem II quantum yield. In a dark-adapted leaf this is equivalent to Fv/Fm. In a light-adapted leaf it is equivalent to Fv‘/Fm‘.
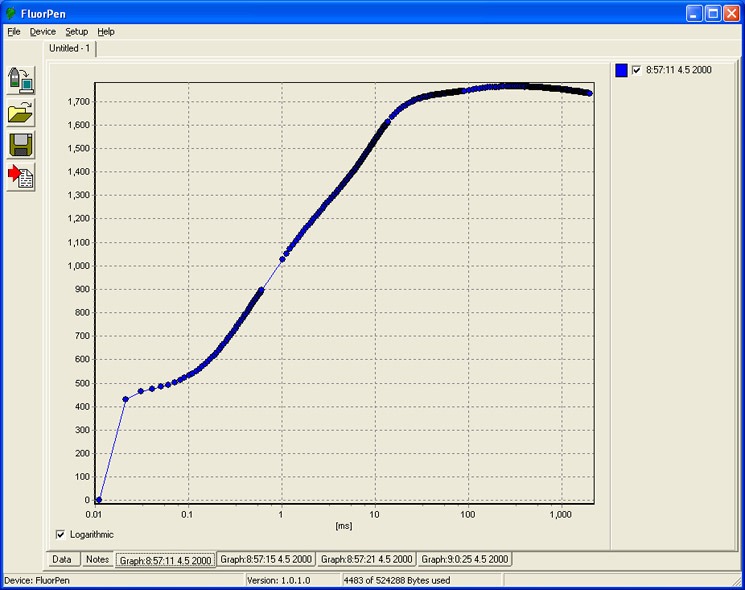
- OJIP – Chlorophyll Fluorescence Induction Kinetics (fast transient analysis) is a simple and non-invasive tool to monitor chloroplast function. Provided OJIP analysis is used as sensitive and reliable fast test for the functionality and vitality of photosynthetic system.
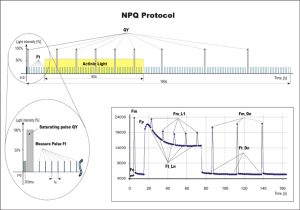
- NPQ – Non-Photochemical Quenching. Provided are two predefined NPQ protocols differing in the duration of light exposure and dark recovery phase as well as in the number of intervals between the pulses. It is typically used for quantification of photochemical and non-photochemical quenching in dark-adapted samples.
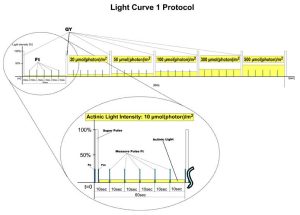
- Light Curve – Adaptation of Quantum Yield to Several Different Light Levels. There are three predefined Light Curve protocols based on pulse modulated fluorometry differing in number and duration of single light phases and light intensities. Light Curve protocols provide successive measurements of the sample photosynthesis under various light intensities of continuous illumination relating the rate of photosynthesis to photon flux density.
Measuring light power is adjustable by flash duration; actinic light is adjustable up to 1000 µmol photons/m2/s and saturating flashes are adjustable up to 3,000 µmol photons/m2/s.
All FluorPens come with two data transfer options built in USB and Bluetooth and they all have a GPS module built in. The FluorPen operates on Lithium battery that can be easily recharged via USB port.
Z991 FluorPen is available with two style of leaf clip. Standard leaf clip is attached to the device, and the removable leaf clip can be placed on the leaf ahead of time for dark adaptation purposes. An optional feature of the FluorPen is the PAR light meter (LM). When included it is built in on the front panel of the device and it measures photosynthetically active radiation in 400-700nm range in units of umol photons/m2/s. The list of available versions is as follows:
Z991 Standard leaf clip FluorPen
Z991-D FluorPen adapted for use with detachable leaf clips (10 leaf clips included)
Z991-LM Standard leaf clip FluorPen with PAR light meter built in
Z991-D-LM FluorPen adapted for use with detachable leaf clips (10 leaf clips included) and built in PAR light meter
In addition to the 4 standard Z991 FluorPen versions, two Monitoring Pen FluorPens are also available. These devices measure the same parameters as the standard FluorPens but are designed for use in different environments. Z991-M-E is the Extended Monitoring Pen designed for extreme weather conditions and long term, unattended monitoring of chlorophyll fluorescence parameters in the field or in the lab. It features rugged weather proof case and may be ordered with features for remote (software control, data collection) or manual two button control and data collection to the device. The second version of the Monitoring Pen Z991-M-A is the Aquatic Monitoring Pen and it has been designed for use under water (up to 2m depth). The control of the Aquatic Monitoring Pen may be with the two buttons by the user or via remote software on the computer (maintained above the water level). a customized version of the Z991-M-A may be provided for depths greater than 2m (contact Qubit Systems for details).
- Hand held PAM chlorophyll Fluorometer
- Different leaf clip options
- Ideal for field, greenhouse and lab research
- Built in GPS module
- Rechargabel lithium battery
- Both USB and BT data communication via PC software
- Photosynthesis research and teaching
- Plant screening and phenotyping
- Plant stress responses
- Herbicide research
- Agronomy and forestry research
- Measured/Calculated Parameters: F0 , FT , FM , FM ‘ , QY, OJIP*, NPQ 1,2*, and Light Curve 1,2,3*
- Saturating Light:
Adjustable from 0 to 100 % (up to 3,000 µmol(photon).m-2.s-1 - Actinic Light:
Adjustable from 0 to 100 % (up to 1,000 µmol(photon)/m2/s - Measuring Light:
Adjustable from 0 to 100 % (up to 0.09 µmol(photon)/m2 per pulse) - Emitter:
Blue 470 nm LED – optically filtered and precisely focused - Detector Wavelength Range: PIN photodiode with 697 to 750 nm bandpass filters
- FluorPen 1.0 Software:
Windows 2000, XP, or higher** - Both USB and BT data transfer
- built in GPS module
- Memory Capacity (16 Mb):
Ft: up to 149,000 measures
QY: up to 95,000 measues
LC1: up to 3,000 measures
LC2: up to 3,500 measures
LC3: up to 2,600 measures
NPQ1: up to 800 measures
NPQ2: up to 500 measures
OJIP: up to 1,100 measures
Internal Data Logging:
Up to 100,000 data points - Display:
2 x 8 characters LC display - Keypad:
Sealed, 2-key tactile response - Keypad Escape Time:
Turns off after 5 minutes of no use - Power Supply:
rechargabel Lithium battery - Low Battery Detection:
Low battery indication displayed - Size:120 mm x 57 mm x 30 mm 4.7″ x 2.2″ x 1.2″
- Weight:180 g, 6.5 oz
- Sample Holder:
Mechanical leaf clip – standard, open, or detachable - Operating Conditions:
Temperature: 0 to 55 ºC; 32 to 130 ºF Relative humidity: 0 to 95 % (non-condensing) - Storage Conditions:
Temperature: -10 to +60 ºC; 14 to +140 ºF Relative humidity: 0 to 95 % (non-condensing) - Warranty:
1 year parts and labor
- PTUSHENKO V. V., AVERCHEVA O. V., BASSARSKAYA E. M., BERKOVICH Y. A., EROKHIN, A. N., SMOLYANINA S. O., and ZHIGALOVA, T. V. (2015). Possible reasons of a decline in growth of Chinese cabbage under a combined narrowband red and blue light in comparison with illumination by high-pressure sodium lamp. Scientia Horticulturae, 194: pp. 267-277, doi:10.1016/j.scienta.2015.08.021
- OREKHOV D.I., V., YAKOVLEVA S. N., GORYACHEV F. F., PROTOPOPOV F.F., and ALEKSEEV, A.A. (2015). The use of parameters of Chlorophyll a fluorescence induction to evaluate the state of plants under anthropogenic load. Biophysics, 2015, Vol. 60, No. 2, pp. 263–268, doi: 10.1134/S0006350915020128
- FESENKO I. A., ARAPIDI G. P., SKRIPNIKOV A. Y., ET AL. (2015). Specific pools of endogenous peptides are present in gametophore, protonema, and protoplast cells of the moss Physcomitrella patens. Pesticide Biochemistry and Physiology. Volume 15, Pages 1-16. DOI 10.1186/s12870-015-0468-7
- HUMPLÍK J. F., LAZÁR D., FÜRST T. ET AL. (2015). Automated integrative high-throughput phenotyping of plant shoots: a case study of the cold-tolerance of pea (Pisum sativumL.). Plant Methods. Volume 11, Pages 1-11. DOI 10.1186/s13007-015-0063-9
- TRIPATHI D. K., SINGH V. P., PRASAD S. M. ET AL. (2015). Silicon-mediated alleviation of Cr(VI) toxicity in wheat seedlings as evidenced by chlorophyll florescence, laser induced breakdown spectroscopy and anatomical changes. Ecotoxicology and Environmental Safety, Volume 113, Pages 133-144. DOI:10.1016/j.ecoenv.2014.09.029
- AJIGBOYE O. O., MURCHIE E., RAY R. V. (2014). Foliar application of isopyrazam and epoxiconazole improves photosystem II efficiency, biomass and yield in winter beat. Pesticide Biochemistry and Physiology. Volume 114, Pages 52–60. DOI:10.1016/j.pestbp.2014.07.003
- CALDERÓN R., LUCENA C., TRAPERO-CASAS J. L. ET. AL. (2014). Soil temperature determines the reaction of olive cultivars to Verticillium dahliae pathotypes. PLoS One. Volume 9. DOI:10.1371/journal.pone.0110664, http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0110664
- JUETERBOCK A., KOLLIAS S., SMOLINA I. ET AL. (2014). Thermal stress resistance of the brown alga Fucus serratusalong the North-Atlantic coast: Acclimatization potential to climate change. Marine Genomics. Volume 13, Pages 27-36. DOI:10.1016/j.margen.2013.12.008, http://www.sciencedirect.com/science/article/pii/S1874778713000871
- PTUSHENKO V. V., PTUSHENKO O. S. AND TIKHONOV A. N. (2014) Chlorophyll Fluorescence Induction, Chlorophyll Content, and Chromaticity Characteristics of Leaves as Indicators of Photosynthetic Apparatus Senescence in Arboreous Plants. Biochemistry (Moscow). Volume 79, Issue 3, Pages 260-272. DOI: 10.1134/S0006297914030122
- RASOOL B., KARPINSKA B., KONERT G. ET AL. (2014). Effects of light and the regulatory B-subunit composition of protein phosphatase 2A on the susceptibility of Arabidopsis thaliana to aphid (Myzus persicae) infestation. Frontiers in Plant Science. Volume 5. DOI: 10.3389/fpls.2014.00405, http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4140078/
- THWE A. A. AND KASEMSAP P. (2014). Quantification of OJIP Fluorescence Transient in Tomato Plants Under Acute Ozone Stress. Kasetsart Journal: Natural Science, Volume 48, Page 665 – 675.
- AROCA R., RUIZ-LOZANO M. J., ZAMARREÑO A. M., ET AL. (2013). Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. Journal of Plant Physiology, Volume 170, Issue 1, Pages 47-55. DOI:10.1016/j.jplph.2012.08.020
- ESTRADA B., AROCA R., BAREA J. M. ET. AL. (2013). Native arbuscular mycorrhizal fungi isolated from a saline habitat improved maize antioxidant systems and plant tolerance to salinity. Plant Science. Volume 201-202, Pages 42-51. DOI:10.1016/j.plantsci.2012.11.009, http://www.sciencedirect.com/science/article/pii/S0168945212002427
- GAJEWSKA E., DROBIK D., WIELANEK M. ET AL. (2013). Alleviation of nickel toxicity in wheat (Triticum aestivum L.) seedlings by selenium supplementation. Biological Letters. Volume 50, Issue 2, Pages 65–78. DOI: 10.2478/biolet-2013-0008
- PTUSHENKO V.V., PTUSHENKO E. A., SAMOILOVA O. P. ET AL. (2013). Chlorophyll fluorescence in the leaves of TRADESCANTIA species of different ecological groups: Induction events at different intensities of actinic light. Biosystems. Volume 114, Issue 2, Pages 85–97. DOI:10.1016/j.biosystems.2013.08.001
- SHAKHATREH Y., CARVALHO P., FOULKES J. ET AL. (2013). ACLIMAS annual meeting – Rabat, 23 October 2013. http://www.aclimas.eu/Reports-Document/ACLIMAS%20SWIM-DP%202nd%20Annual%20Meeting,%20Rabat,%2023-24%20October%202013/ACLIMAS,%20Jordan%20achievements%20and%20future%20plans.pdf
- VREDENBERG W. AND PAVLOVIČ A. (2012). Chlorophyll a fluorescence induction (Kautsky curve) in a Venus flytrap (Dionaea muscipula) leaf after mechanical trigger hair irritation. Journal of Plant Physiology. Volume 170, Pages 242-250. DOI:10.1016/j.jplph.2012.09.009
- KLEM K., AČ A., HOLUB P. ET AL. (2012). Interactive effects of PAR and UV radiation on the physiology, morphology and leaf optical properties of two barley varieties. Environmental and Experimental Botany. Volume 75, Pages 52-64. DOI:10.1016/j.envexpbot.2011.08.008, http://www.sciencedirect.com/science/article/pii/S0098847211001900
- CHYTYK, C. J., HUCL, P. J. AND GRAY, G. R. (2011). Leaf photosynthetic properties and biomass accumulation of selected western Canadian spring wheat cultivars. Canadian Journal of Plant of Science. Volume 91, Pages 305-314. DOI: 10.4141/CJPS09163
- COWLEY R. AND LUCKETT D. (2011) Chlorophyll fluorescence as a method to detect moisture-limiting stress in canola. 17thAustralian Research Assembly on Brassicas (ARAB)
- KOCUREK V., VONDRA M. AND SMUTNÝ, V. (2011). Efficacy of reduced doses of bentazone assessed by instruments based on measurement of chlorophyll fluorescence. Acta Universitatis Agriculturae et Silviculturae Mendelianae Brunensis. Volume 59, Pages 137-144. DOI: 10.11118/actaun201159010137
- KUVYKIN V., PTUSHENKO V. V., VERSHUBSKII A. V. ET AL. (2011).Regulation of electron transport in C3 plant chloroplasts in situ and in silico: Short-term effects of atmospheric CO2 and O2. Biochimica et Biophysica Acta (BBA) – Bioenergetics, Volume 1807, Issue 3, Pages 336-347. DOI:10.1016/j.bbabio.2010.12.012
- LUCIŃSKI R., MISZTAL L. SAMARDAKIEWICZ S. ET AL. (2011). The thylakoid protease Deg2 is involved in stress-related degradation of the photosystem II light-harvesting protein Lhcb6 inArabidopsis thaliana New Phytologist. Volume 192, Pages 74-86. DOI: 10.1111/j.1469-8137.2011.03782.x.
- RUÍZ-SÁNCHEZ, M., ARMADA, E., MUÑOZ, Y., ET AL. (2011). Azospirillum and arbuscular mycorrhizal colonization enhance rice growth and physiological traits under well-watered and drought conditions. Journal of Plant Physiology. Volume 168, Issue 10, Pages 1031-1037. DOI:10.1016/j.jplph.2010.12.019
- SAMOILOVA O. P., PTUSHENKO V. V., KUVYKIN V. ET AL. (2011) Effects of light environment on the induction of chlorophyll fluorescence in leaves: A comparative study of Tradescantia species of different ecotypes. Biosystems. Volume 105, Issue 1, Pages 41–48. DOI:10.1016/j.biosystems.2011.03.003
- CESSNA S., DEMMIG-ADAMS B. AND ADAMS III W. W. (2010). Exploring Photosynthesis and Plant Stress Using Inexpensive Chlorophyll Fluorometers. Journal of Natural Resources and Life Sciences Education. Volume 39, Pages 22-30. DOI: 10.4195/jnrlse.2009.0024u
- FERNANDEZ-MARIN B., BECERRIL J. M. AND GARCIA PLAZAOLA J. I. (2010). Unravelling the roles of desiccation-induced xanthophyll cycle activity in darkness: A case study in Lobaria pulmonaria. Planta. Volume 231, Pages 1335-1342. DOI: 10.1007/s00425-010-1129-6
- PAVLOVIČ A., SLOVÁKOVÁ L., PANDOLFI C. ET AL. (2010). On the mechanism underlying photosynthetic limitation upon trigger hair irritation in the carnivorous plant Venus flytrap (Dionaea muscipula Ellis). Journal of Experimental Botany, Volume 62, Pages 1991–2000. DOI: 10.1093/jxb/erq404
- RUIZ-SÁNCHEZ M., AROCA R., MUÑOZ Y., ET AL. (2010). The arbuscular mycorrhizal symbiosis enhances the photosynthetic efficiency and the antioxidative response of rice plants subjected to drought stress. Journal of Plant Physiology. Volume 167, Pages 862-869. DOI: 10.1016/j.jplph.2010.01.018
- HARDING S. A., JARVIE M. M., LINDROTH R. L. ET AL. (2009). A comparative analysis of phenylpropanoid metabolism, N utilization, and carbon partitioning in fast- and slow-growing POPULUS hybrid clones. Journal of Experimental Botany. Volume 60, Pages 3443-3452. DOI:10.1093/jxb/erp180
- KUVYKIN I.V., VERSHUBSKII A.V., PRIKLONSKII V.I. ET AL. (2009). Computer simulation study of pH-dependent regulation of electron transport in chloroplasts. Biophysics. Volume 54, Pages 455-464. DOI: 10.1134/S0006350909040101
- MACEK P., MACKOVÁ J. AND DE BELLO F., (2009). Morphological and ecophysiological traits shaping altitudinal distribution of three Polylepis treeline species in the dry tropical Andes. Acta Oecologica, Volume 35, Pages 778–785. DOI:10.1016/j.actao.2009.08.013
- ROSESCU M. R. AND ANDREI M. (2009). The study of photosystem II efficiency on selected synanthrophic plant species. Annals Food Science and Technology. Volume 10, Pages 115-119.
- BARTÁK, M (2008) Biophysical Methods and Approaches to Monitor In-situ Lichen Responses to Environmental Extremes. Coordination Action for Research Activities on life in Extreme Environments. Publication 2.
- KLEM K. AND BAJEROVA, E., (2008). Adjustment of herbicide dose in sugar beet based on non-invasive chlorophyll fluorescence measurements. Agricultural And Biosystems Engineering For A Sustainable World: National Conference On Agricultural Engineering, Hersonissos, Crete, Greece, Pages 23-25.
- WOO N. S., BADGER M. R. AND POGSON B. J. (2008) A rapid, non-invasive procedure for quantitative assessment of drought survival using chlorophyll fluorescence Plant Methods, Volume 4, Issue 27, Pages 1-14. DOI:10.1186/1746-4811-4-27
- Bckg = background
- F0: = F50µs; fluorescence intensity at 50 µs
- FJ: = fluorescence intensity at j-step (at 2 ms)
- Fi: = fluorescence intensity at i-step (at 60 ms)
- FM: = maximal fluorescence intensity
- FV: = FM – F0 (maximal variable fluorescence)
- VJ = (FJ – F0) / (FM – F0)
- Vi = (Fi – F0) / (FM – F0)
- FM / F0
- FV / F0
- FV/ FM
- M0 or (dV / dt)0 = TR0 / RC – ET0 / RC = 4 (F300 – F0) / (FM – F0)
- Area = area between fluorescence curve and FM (background subtracted)
- Fix Area = total area above the OJIP fluorescence transient – between F40µ and F1s(background subtracted)
- SM = area / FM – F0 (multiple turn-over)
- Ss = the smallest Sm turn-over (single turn-over)
- N = SM . M0 . (1 / VJ) turn-over number QA
- Phi_P0 = 1 – (F0 / FM (or FV / FM)
- Psi_0 = 1 – VJ
- Phi_E0 = (1 – F0 / FM)) . Psi_0
- Phi_D0 = 1 – Phi_P0 – (F0 / FM)
- Phi_Pav = Phi_P0 – (SM / tFM); tFM) = Time to reach FM (in ms)
- ABS / RC = M0 . (1 / VJ) . (1 / Phi_P0)
- TR0 / RC = M0 . (1 / VJ)
- ET0 / RC = M0 . (1 / VJ) . Phi_0)
- DI0 / RC = (ABS / RC) – (TR0 / RC)

NPQ and Light Curve Protocols
NPQ Protocol Includes Five Measurements in Actinic Light and three measurements during dark relaxation.
NPQ 1 protocol: light duration 60s, 5 pulses; dark recovery duration 88s, 3 pulses
NPQ 2 protocol: light duration 200s, 10 pulses; dark recovery duration 390s, 7 pulses
NPQ_Ln = (FM – FM_Ln) / FM_Ln
NPQ_Lss = (FM – FM_Lss) / FM_Lss
NPQ_Dn = (FM – FM_Dn) / FM_Dn
Formulas Derived From:
R.J. Strasser, A. Srivastava and M. Tsimilli-Michael (2000): The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Probing Photosynthesis: Mechanism, Regulation and Adaptation (M. Yunus, U. Pathre and P. Mohanty, eds.), Taylor and Francis, UK, Chapter 25, pp 445-483.