The Z100 FluorCam – Closed System is a chlorophyll fluorescence imaging system designed for non-invasive studies of photosynthetic parameters in plants and algae via chlorophyll fluorescence.
The Closed FluorCam System Z100 consists of a high-resolution CCD camera (720×560 pixels), 4 fixed LED panels that illuminate the sample, imaging software with protocols, and 7 position filter wheel (optional) if imaging of other proteins’ fluorescence (i.e. GFP) is required. The LED panels provide uniform irradiance over samples up to 10 x 10 cm – suitable for small plants, detached leaves, Petri plates with seedlings or algal cultures, etc. The system allows dark adaptation and includes a high-performance PC and a comprehensive software package
- High resolution CCD camera based imaging system
- 4 LED panels for standard excitation
- Optional additional LED panel available around the CCD camera
- User friendly software with protocols for Fv/Fm, Kautsky induction, Quenching analysis, Light Curve, steady state fluorescence (standard)
- Optional protocols for QA re-oxidation, Fast fluorescence induction (OJIP) with 1µs resolution, PAR absorptivity and NDVI reflectance index
- Measurements of up to 50 different chlorophyll fluorescence paramters
- Imaging of other fluorescent proteins (GFP, YFP etc) with optional filter wheel, filters, and LED panels
- Includes dark adaptation chamber
- Chlorophyll Fluorescence Imaging: Plants and Algae
- Photosynthetic Research
- Biotic and Abiotic Stress Resistance in Plants
- Plant Pathogen Research
- Stomatal Patchiness
- Growth and Yield Improvements in Plants
- Fluorescence parameters: (F0, FM, FV, F0’, FM’, FV’, QY(II)), Abs PAR-value, or the parameters that are calculated from fluorescence emission (e.g., NPQ, FV/FM, FV’/FM’, Rfd, qN, qP), PAR-absorptivity, photosynthetic electron transport rate (PS), and others
- Excitation light sources: Standard: red-orange (617 nm), cool white (typical colour temperature 6500 K) Optional: royal-blue (447 nm), blue (470 nm), green (530 nm), cyan (505 nm), red (627 nm), deep-red (655 nm), amber (590 nm)
- Saturating pulses intensity: 4,000 µmol(photons)/m²/s (in a standard version), 6,000 µmol(photons)/m²/s (in the light-upgraded version)
- Actinic light intensity: Up to 2,000 µmol(photons)/m²/s (in standard), up to 3,000 µmol(photons)/m²/s (in light upgraded version)
- Filter wheel: 7 positions
- Light regime: Static or dynamic (sinus form)
- Custom defined protocols: Variable timing, special language and scripts
- CCD detector wavelength range: 400 – 1000 nm
- CCD format: 720 x 560 pixels; optionally 1024 x 768 pixels (video mode) or 1392 x 1040 pixels (snapshot mode)
- Imaging frequency: Maximum 50 frames per second
- pixel size: 8.2 µm x 8.4 µm; optionally 6.45 µm x 6.45 µm
- A/D bit resolution: 12 bit
- Spectral response: QE max at 540 nm (~70 %), 50 % roll-off at 400 nm and 650 nm
- Read-out noise: Less than 12 electrons RMS – typically only 10 electrons
- Full-well capacity: Greater than 70,000 electrons (unbinned)
- Bios: upgradeable firmware
- Communication port: USB 2.0
- Outer dimensions: 471 mm (W) x 473 mm (D) x 512 mm (H)
- Weight: 40 kg
- Power input: 1100 W
- Electrical: 90 -240V
- PERIN G., SEGALLA A., BASSO S. et al. (2015): Biotechnological Optimization of Light Use Efficiency in NannochloropsisCultures for Biodiesel Production. Chemical Engineering Transactions. Volume 37, Pages 763-768. DOI: 10.3303/CET1437128
- PERIN G., BELLAN A., SEGALLA A., et al. (2015): Generation of random mutants to improve light-use efficiency of Nannochloropsis gaditana cultures for biofuel production. Biotechnology for Biofuels 8: p. 161. DOI: 10.1186/s13068-015-0337-5
- BOURDAIS G., BURDIAK P., GAUTHIER A., ET AL. (2015): Insights from the cold transcriptome of Physcomitrella patens: global specialization pattern of conserved transcriptional regulators and identification of orphan genes involved in cold acclimation. The New Phytologist. Volume 205, Pages 869-881. DOI:10.1111/nph.13004
- VERCRUYSSEN L., TOGNETTI V.B., GONZALES N. et al. (2015): GROWTH REGULATING FACTOR5 Stimulates Arabidopsis Chloroplast Division, Photosynthesis, and Leaf Longevity.Plant Physiology 167(3): 817-32. doi: 10.1104/pp.114.256180
- BEIKE A.K., LANG D., ZIMMER A.D. et al. (2015): Insights from the cold transcriptome of Physcomitrella patens: global specialization pattern of conserved transcriptional regulators and identification of orphan genes involved in cold acclimation. The New Phytologist 205(2): 869-881. DOI:10.1111/nph.13004
- DOOLEY F. D.,WYLLIE-ECHEVERRIA S., GUPTA E., ET AL. et al. (2015): Tolerance of Phyllospadix scouleri seedlings to hydrogen sulfide. Aquatic Botany. Volume 123, Pages 72–75. DOI: 10.1016/j.aquabot.2015.02.004
- HURA K., HURA T., GRZESIAK M. et al. (2014): Function of the photosynthetic apparatus of oilseed winter rape under elicitation by Phoma lingam phytotoxins in relation to carotenoid and phenolic levels. Acta Physiol Plant. 36: 295–305. doi:10.1007/s11738-013-1410-y
- LEAL M.C., JESUS B., EZEQUIEL J. et al. (2014): Concurrent imaging of chlorophyll fluorescence, Chlorophyll a content and green fluorescent proteins-like proteins of symbiotic cnidarians. Marine Ecology 1:13. DOI: 10.1111/maec.12164
- ALINIAEIFARD S. and VAN MEETEREM U. (2014): Natural variation in stomatal response to closing stimuli among Arabidopsis thaliana accessions after exposure to low VPD as a tool to recognize the mechanism of disturbed stomatal functioning. Journal of Experimental Botany 65(22): 6529-6542. DOI:10.1093/jxb/eru370
- HIDA E., CAKO V., BABANI F. et al. (2014): The Influence of Stress Analyzed By The Emitted Fluorescence Changes. IOSRJEN. 2014; 4(6): 38-43.
- HIDA E., CAKO V., BABANI F. et al. (2014): Activity Imaging Photosynthetic Of Populus X Canadensis Moench Plants In Air Pollution. International Journal of Engineering Inventions. Volume 3. Pages 35-40.
- GAWROŃSKI P, WITOŃ D, VASHUTINA K. et al. (2014): Mitogen-Activated Protein Kinase 4 Is a Salicylic Acid-Independent Regulator of Growth But Not of Photosynthesis in Arabidopsis. Molecular Plant. Volume 7, Pages 1151–1166. DOI: http://dx.doi.org/10.1093/mp/ssu060
- BEELER S., LIU H., STADLER M., et al. (2014): Plastidial NAD-Dependent Malate Dehydrogenase Is Critical for Embryo Development and Heterotrophic Metabolism in Arabidopsis. Plant Physiology. Volume 164, Pages 1175–1190. DOI: 10.1104/pp.113.233866
- JOHNSON, S. M., LIM F. L., FINKLER A. et al. (2014): Transcriptomic analysis of Sorghum bicolor resp onding to combined heat and drought stress. Plant Physiology. BMC genomics.,Volume 15, Page 456. DOI: 10.1186/1471-2164-15-456
- LEE S. B., YOO S. Y., KIM D. Y. et al. (2014): Proteomic evaluation of the response of soybean (Glycine max var Seoritae) leaves to UV-B. Plant Omics.
FluorCam 7 Software
- Automated experimental protocols via a Windows Wizard.
- Multiple (automatically repeated) experiments.
- Automated labeling of individual plants, or samples, within the field of view.
- Kinetic analysis of data from all samples within the field of view.
- Numerous image manipulation tools.
- Barcode reader support (Optional).
- Export to text files, avi, bmp or raw data formats.
- Windows 2000, XP, Vista, W7 compatible
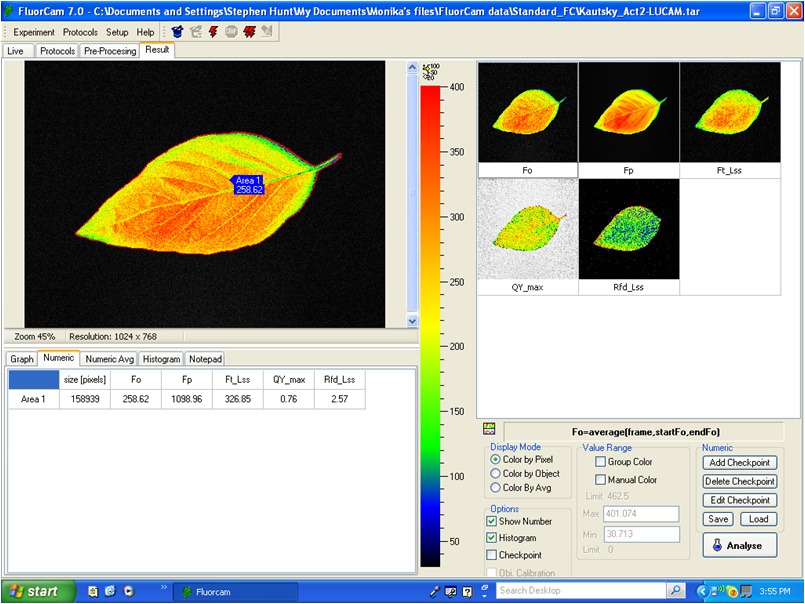
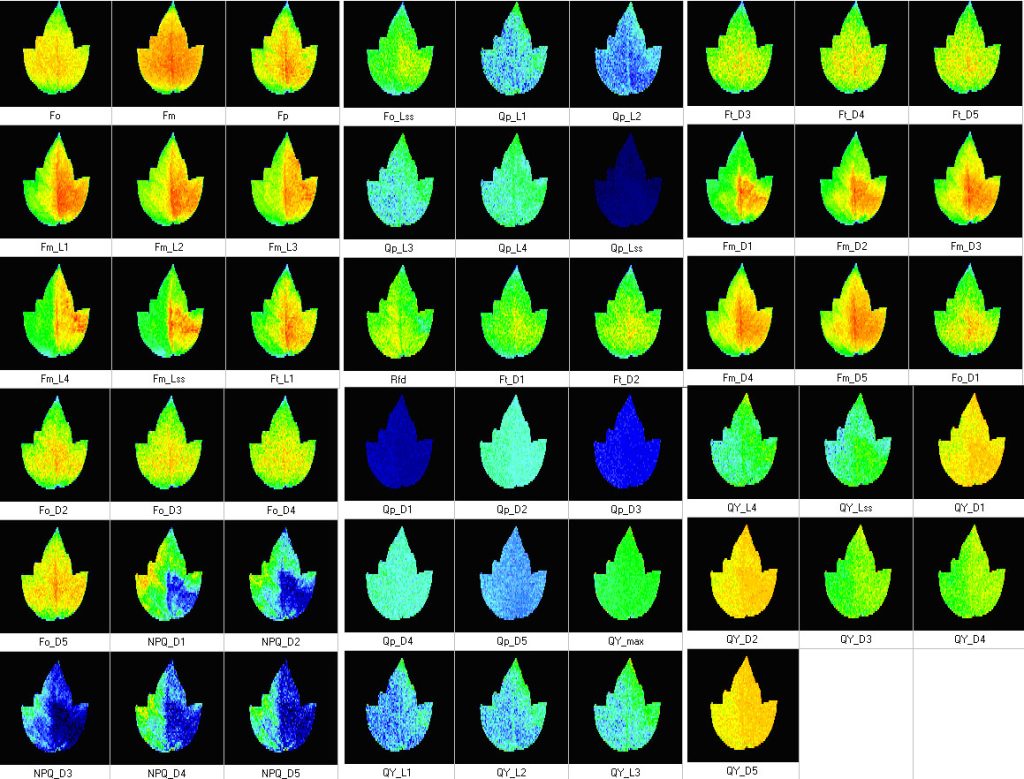
Measured Parameters:
- Fv/Fm
- Kautsky induction
- Quenching analysis
- steady state fluorescence eg. ChlF, GFP and other FPs (filter wheel required)
- QA re-oxidation (needs optional electronic module)
- Fast fluorescence induction (OJIP) with 1µs resolution (needs optional electronic module)
- PAR absorptivity (needs optional accessories: filter wheel and additional IR LED panel)
- Measured parameters: FO, FM, FV, FO’, FM’, FV’, FT
- More than 50 calculated parameters: FV/FM, FV’/FM’, PhiPSII , NPQ, qN, qP, Rfd, PAR-absorptivity coefficient, electron transport rate (ETR), and many others
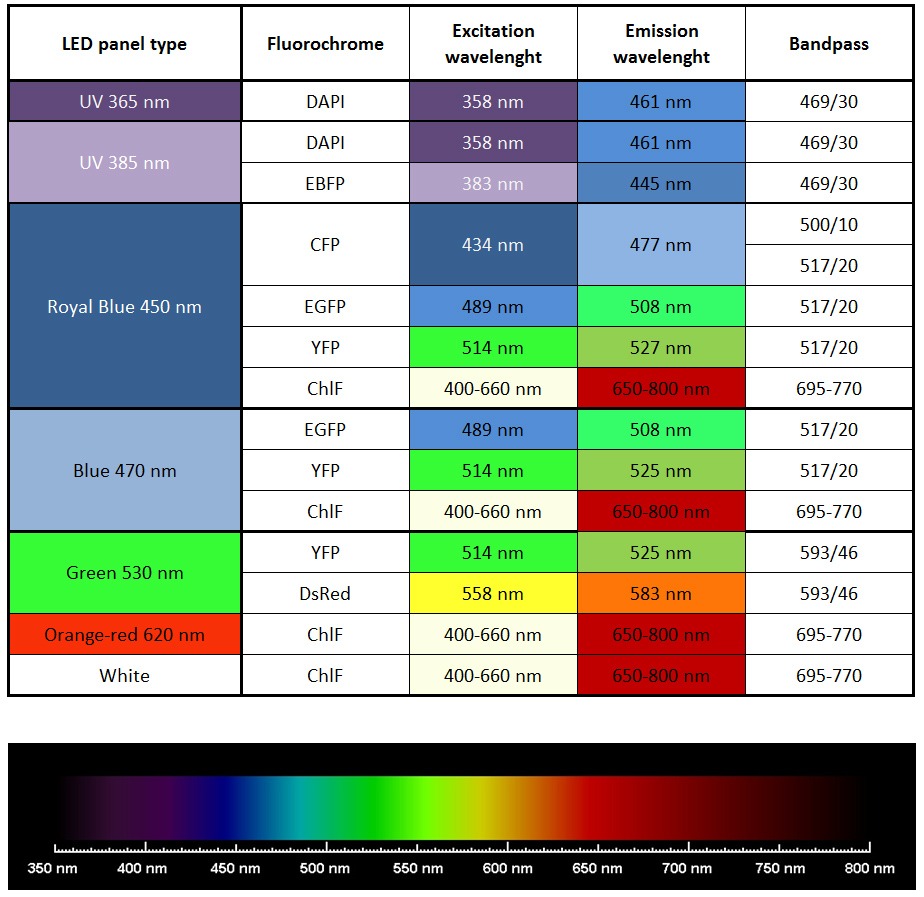
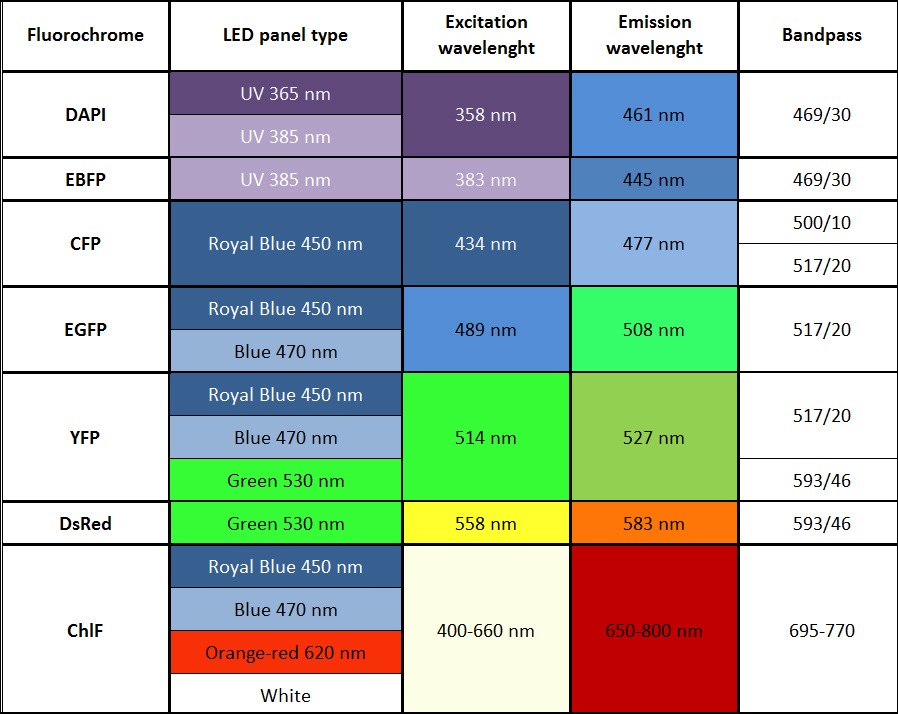
List of most common fluorochromes and types of suitable excitation LED panels