The Z200 Open- FluorCam System is a highly modular instrument for chlorophyll fluorescence imaging of plants, plant tissues, and algae. The system is composed of 4 LED light panels, a CCD camera, and controller, an adjustable frame, and software with a PC computer. This system is available in standard 10×10 and 20×20 imaging areas.
The LED panels and the light source generating saturating flashes can be arranged at various angles and distances from the sample. The position of the camera may also be adjusted for added precision. The maximum area for imaging with standard hardware is 10 x 10 cm, or 20 x 20 cm, depending on the size of selected light sources. Much larger areas, up to 200 x 100 cm, can be imaged by the addition of LED panels and/or by mounting the whole system on a movable frame.
- High resolution CCD camera based imaging system
- 4 LED panels for standard excitation
- Optional additional LED panel available around the CCD camera
- User friendly software with protocols for Fv/Fm, Kautsky induction, Quenching analysis, Light Curve, steady state fluorescence (standard)
- Optional protocols for QA re-oxidation, Fast fluorescence induction (OJIP) with 1µs resolution, PAR absorptivity and NDVI reflectance index
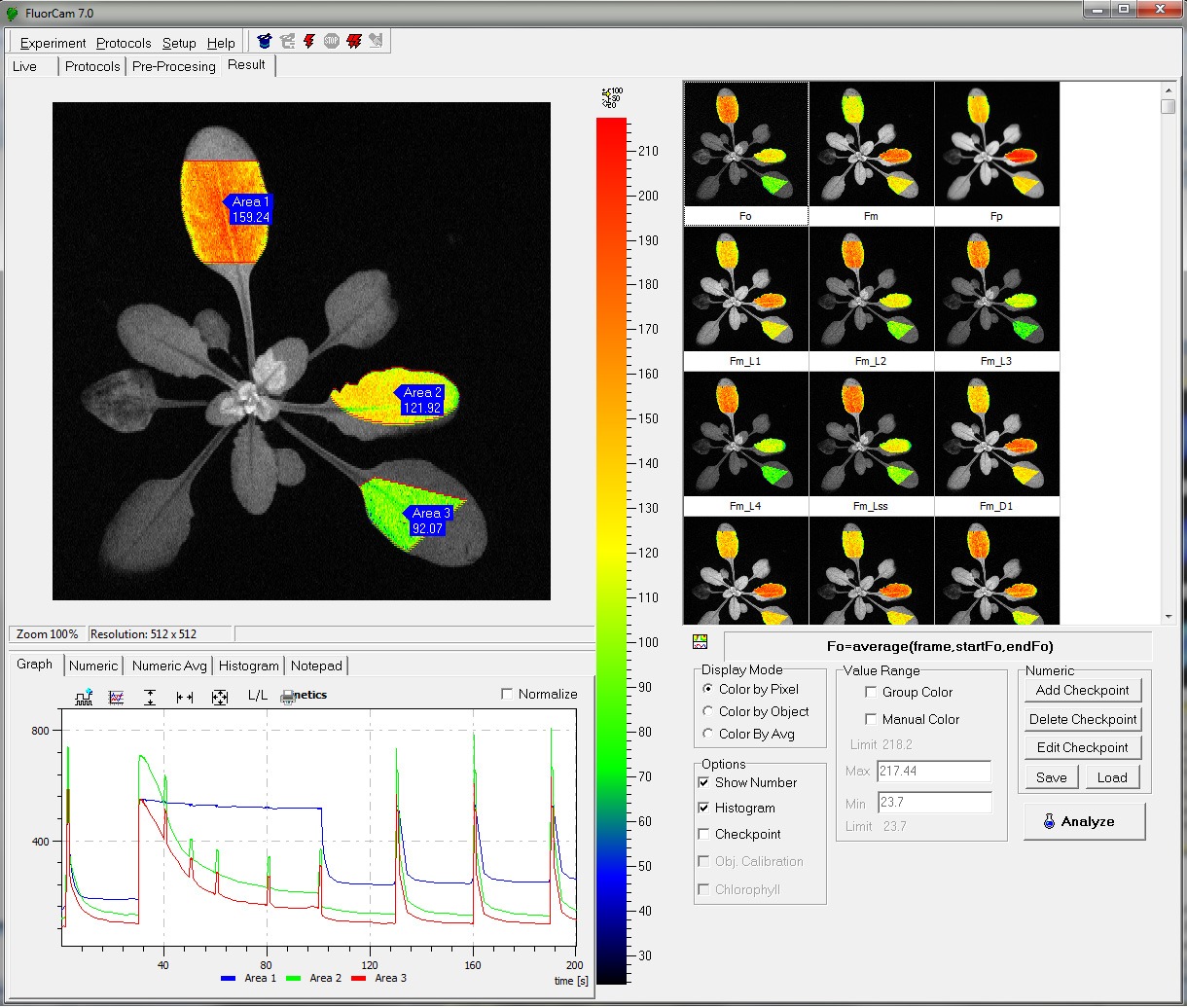
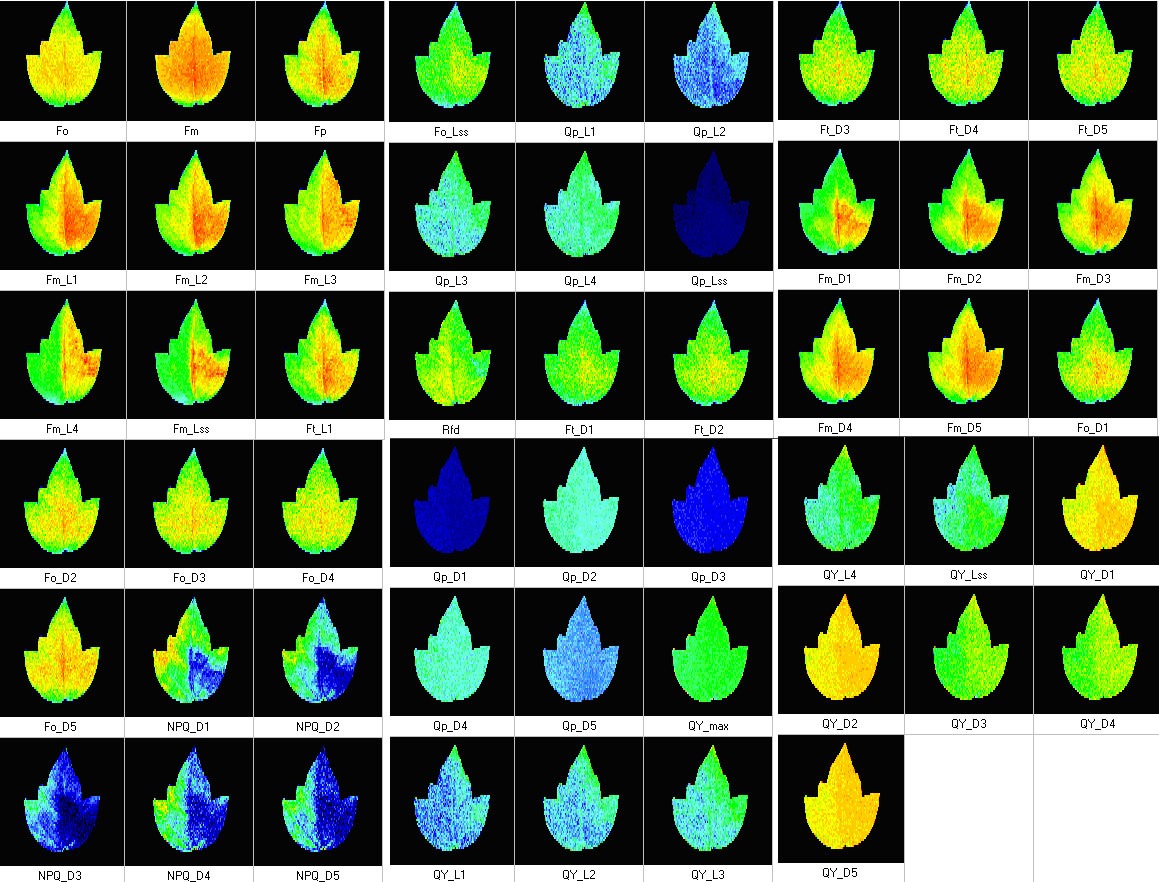
- Measurements of up to 50 different chlorophyll fluorescence paramters
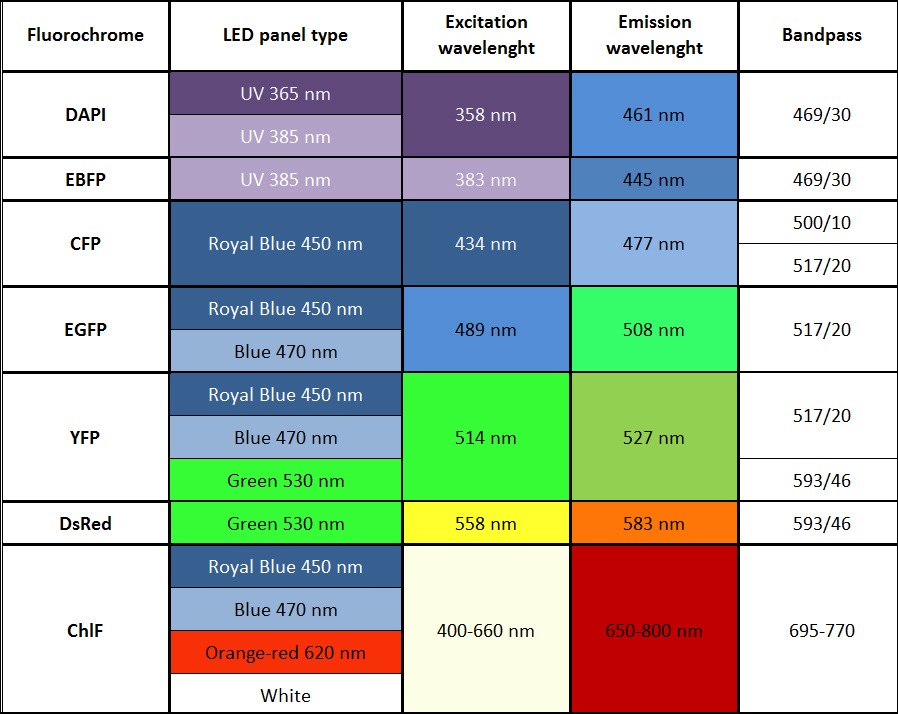
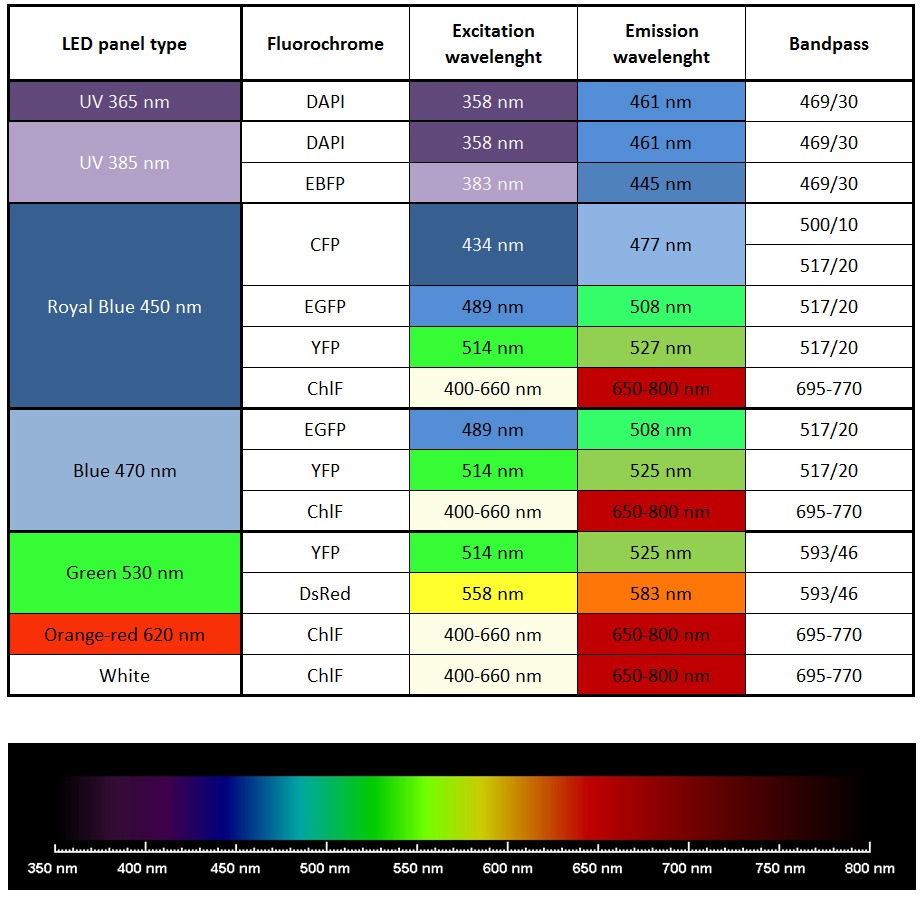
- Imaging of other fluorescent proteins (GFP, YFP etc) with optional filter wheel, filters, and LED panels
- Flexible construction allows for imaging of different size plants
- Chlorophyll Fluorescence Imaging: Plants and Algae
- Photosynthetic Research
- Biotic and Abiotic Stress Resistance in Plants
- Plant Pathogen Research
- Stomatal Patchiness
- Growth and Yield Improvements in Plants
- Fluorescence Parameters:
– Measured parameters: FO, FM, FV, FO’, FM’, FV’, FT
– More than 50 calculated parameters: FV/FM, FV’/FM’, PhiPSII , NPQ, qN, qP, Rfd, PAR-absorptivity coefficient, and many others - Light Sources:
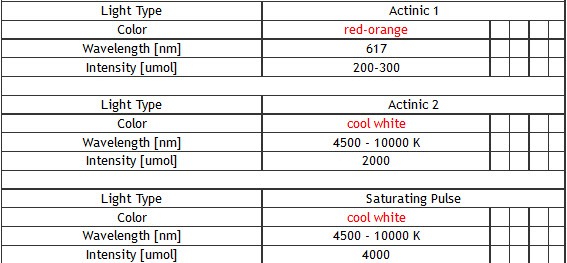
Standard: red-orange (617 nm), cool white (typical colour temperature 6500 K)
Optional: royal-blue (447 nm), blue (470 nm), green (530 nm), cyan (505 nm), red (627 nm), deep-red (655 nm), amber (590 nm) - Super Pulse Intensity:
– 4,000 µmol(photons)/m².s (in standard version)
– 6,000 µmol(photons)/m².s (in light-upgraded version) - Actinic Light Intensity:
Up to 2,000 µmol(photons)/m².s (in standard version)
Up to 3,000 µmol(photons)/m².s (in light-upgraded version) - Filter Wheel:
7 positions - Light Regime:
Static or dynamic (sinus form) - Custom-Defined Protocols:
Variable timing, special language and scripts - CCD Detector Wavelength Range:
400 – 1000 nm - CCD Format:
720 x 560 pixels; optionally 1024 x 768 pixels (video mode) or 1392 x 1040 pixels (snapshot mode) - Pixel Size:
8.2 µm x 8.4 µm; optionally 6.45 µm x 6.45 µm - A/D bit Resolution:
12 bit - Spectral Response:
QE max at 540 nm (~70 %), 50 % roll-off at 400 nm and 650 nm - Read-Out Noise:
Less than 12 electrons RMS – typically only 10 electrons - Full-Well Capacity:
Greater than 70,000 electrons (unbinned) - Imaging Frequency:
Maximum 50 frames per second - Bios
Upgradeable firmware - Communication Port
USB 2.0 - Dimension
710 mm (W) x 710 mm (D) x 430 mm (H) - Weight
Appr. 40 kg - Power Input
Appr. 1100 W - Electrical
90 – 240 V
- CASTILLO-LIZARDO M. G., ARAGÓN I. M., CARVAJAL V. et al. (2015): Contribution of the non-effector members of the HrpL regulon, iaaL and matE, to the virulence of Pseudomonas syringae pv. tomato DC3000 in tomato plants. BMC MICROBIOLOGY Volume 15. DOI:10.1186/s12866-015-0503-8
- FAN X., ZHANG J., LI W. et al. (2015): The NdhV subunit is required to stabilize the chloroplast NADH dehydrogenase-like complex in Arabidopsis. The Plant Journal. Volume 82, Pages 221–231. DOI: 10.1111/tpj.12807
- GRANUM E., PÉREZ-BUENO M. L., CALDERÓN C. E. et al. (2015): Metabolic responses of avocado plants to stress induced by Rosellinia necatrix analysed by fluorescence and thermal paging. European Journal of Plant Pathology. Volume 142. DOI: 10.1007/s10658-015-0640-9
- KROPAT J., TOTTEY S., BIRKENBIHL R. P. et al. (2015): A regulator of nutritional copper signaling in Chlamydomonas is an SBP domain protein that recognizes the GTAC core of copper response element. PNAS. Volume 102, Pages 18730–18735. DOI: 10.1073/pnas.0507693102
- DE MARCOS A., TRIVIÑO M., PÉREZ-BUENO M. L. et al. (2015): Transcriptional profiles of Arabidopsis stomataless mutants reveal developmental and physiological features of life in the absence of stomata. Frontiers in Plant Science. Volume 6, Page 456. DOI: 10.3389/fpls.2015.00456
- ROUSSEAU C., HUNAULT G., GAILLARD S. et al. (2015): Phenoplant: a web resource for the exploration of large chlorophyll uorescence image datasets. Plant Methods. Volume 11. DOI 10.1186/s13007-015-0068-4
- ARMBRUSTER U., CARRILLO L.R., VENEMA K., et al. (2014): Ion antiport accelerates photosynthetic acclimation in fluctuating light environments. Nature Communications 5: 5439. DOI:10.1038/ncomms6439
- PÉREZ-BUENO M. L., BARÓN M., GARCÍA-CARNEROS A. B., et al. (2014): Diagnosis of the Infection of Sunflower by Orobanche cumana Using Multicolour Fluorescence paging. Helia. Volume 37, Pages 173–179. DOI:10.1515/helia-2014-0015
- ROUSSEAU C., BELIN E., BOVE E. et al. (2013): High throughput quantitative phenotyping of plant resistance using chlorophyll fluorescence image analysis. Plant Methods 9: 17. DOI:10.1186/1746-4811-9-17
- CISZEWSKI D., ALEKSANDER-KWATERCZAK U., POCIECHA A. et al. (2013): Small effects of a large sediment contamination with heavy metals on aquatic organisms in the vicinity of an abandoned lead and zinc mine. Environmental Monitoring and Assessment 185(12): 9825-9842. DOI:10.1007/s10661-013-3295-z
- SERODIO J., EZEQUIEL J., FROMMLET J. et al. (2013): A Method for the Rapid Generation of Nonsequential Light-Response Curves of Chlorophyll Fluorescence. Plant Physiology 163(3): 1089-1102. DOI:10.1104/pp.113.225243
- LABUZ J., SZTATELMAN O., BANAS A.K. et al. (2012): The expression of phototropins in Arabidopsis leaves: developmental and light regulation. Journal of Exp. Botany 63(4): 1763–1771. DOI:10.1093/jxb/ers061
- BANAS A.K., LABUSZ J., SZTATELMAN O. et al. (2011): Expression of Enzymes Involved in Chlorophyll Catabolism in Arabidopsis Is Light Controlled. Plant Physiology 157(3): 1497-1504. DOI:10.1104/pp.111.185504
- ROHÁČEK K., SOUKUPOVÁ J. AND BARTÁK M. (2008): Chlorophyll fluorescence: A wonderful tool to study plant physiology and plant stress. Reasearch Signpost. Volume 2, Pages 41-104.
- MOSELEY J. L., ALLINGER T., HERZOG S. et al. (2002): Adaptation to Fe‐deficiency requires remodeling of the photosynthetic apparatus. The EMBO Journal. Volume 21, Pages 6709-6720. DOI 10.1093/emboj/cdf666
- Automated experimental protocols via a Windows Wizard.
- Multiple (automatically repeated) experiments.
- Automated labeling of individual plants, or samples, within the field of view.
- Kinetic analysis of data from all samples within the field of view.
- Numerous image manipulation tools.
- Barcode reader support (Optional).
- Export to text files, avi, bmp or raw data formats.
- Windows 2000, XP, Vista, W7 compatible
- Fv/Fm
- Kautsky induction
- Quenching analysis
- steady state fluorescence eg. ChlF, GFP and other FPs (filter wheel required)
- QA re-oxidation (needs optional electronic module)
- Fast fluorescence induction (OJIP) with 1µs resolution (needs optional electronic module)
- PAR absorptivity (needs optional accessories: filter wheel and additional IR LED panel)
- Measured parameters: FO, FM, FV, FO’, FM’, FV’, FT
- More than 50 calculated parameters: FV/FM, FV’/FM’, PhiPSII , NPQ, qN, qP, Rfd, PAR-absorptivity coefficient, electron transport rate (ETR), and many others
- Standard version of the FluorCam system includes four super bright LED panels – size 13 x 13 cm or 20 x 20 cm. One pair of LED panels provides Measuring Light and Actinic Light 1 (red-orange 617 nm – in standard version). Other two panels provide Actinic Light 2 and Saturating Pulse (cool white 6500 K – in standard version).
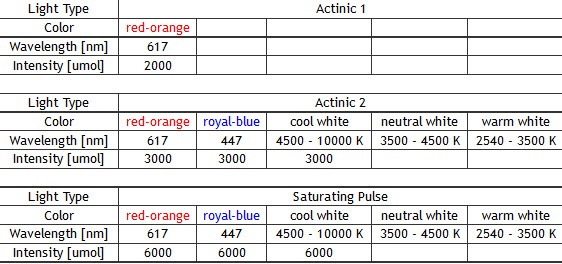
- Other available wavelengths for light sources are: royal-blue (447 nm), blue (470 nm), green (530 nm), cyan (505 nm), red (627 nm), deep-red (655 nm), amber (590 nm) – these lights sources are mounted only in customized FluorCam versions.
- Additional LED panel may be added to increase the colour spectrum of the light sources for multi-colour fluorescence measurements. Additional panel is mounted around the camera. Available colours are: far-red (740 nm), deep-red (660 nm), ultra-violet (365 or 385 nm), green (523 nm), amber (590 nm). Other options available upon request.
- Actinic light intensity:
– 300-2,000 µmol(photons)/m²s in the standard version depending on light wavelength,
– up to 3,000 µmol(photons)/m²s in the light-upgraded version (optional) - Super pulse intensity:
– up to 4,000 µmol(photons)/m²s (in the standard version),
– up to 6,000 µmol(photons)/m²s (in the light-upgraded version (optional) - Single Turnover Flash (STF): 120,000 µmol(photons)/m²s in 100µs pulse (in the QA re-oxidation version)
- Comparison of light intensities reachable by the standard and light upgraded (QA) panels: