CISME stands for Community In Situ Metabolism (pronounced ‘kiss-me’ to reflect the gentle interaction between the instrument and the coral/substrate). A diver-portable respirometer developed to make non-destructive and non-invasive measurements of coral metabolic rates in situ under ambient conditions. The system measures changes in oxygen and pH during short incubations in which water flow and light levels are user controlled. Respiration (R) and photosynthesis (P) rates are calculated from concentration changes in oxygen or carbon dioxide within the enclosed volume.
The CISME system is vital for coral health assessment. It may be used with algae and other low-relief benthic substrates. Respiration and photosynthesis can be determined, both based on O2 and CO2 fluxes, from which RQs and PQs can be calculated. Sample loops provide water samples that can be titrated for total alkalinity to calculate calcification rates. The sample loop can also be used to experimentally introduce substances that might affect metabolism (e.g. acidified seawater for ocean acidification studies).
The CISME system measures oxygen fluxes and changes in pH during in situ incubations in which water flow and light levels are controlled by the user. From changes in concentration respiration rate and photosynthesis rate are calculated. A sample loop provides a water sample that can be titrated for total alkalinity (TA) from which calcification rate is calculated. The sample loop can also be used to conduct experiments in which the user introduces substances that might affect coral metabolism (such as acidified seawater for ocean acidification studies).
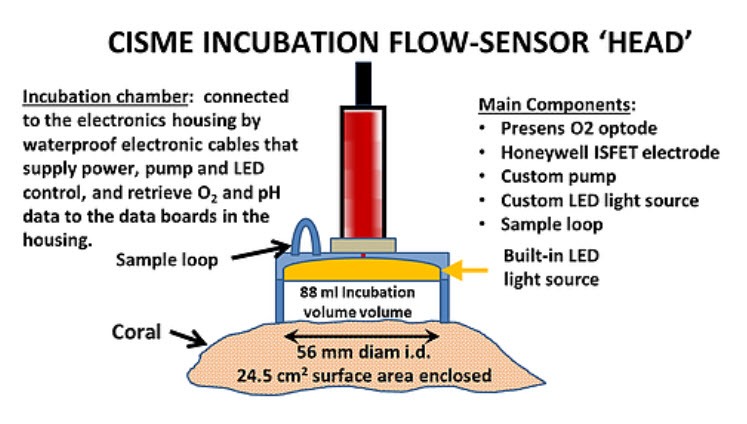
- Electronics components are contained in a pressure resistant acrylic Electronics Housing (80 m depth) connected by waterproof cables to the incubation flow-sensor “head”.
- An Incubation Head housing the sensors, pump, LED light source is attached by the user to the coral/substrate surface with special retractors.
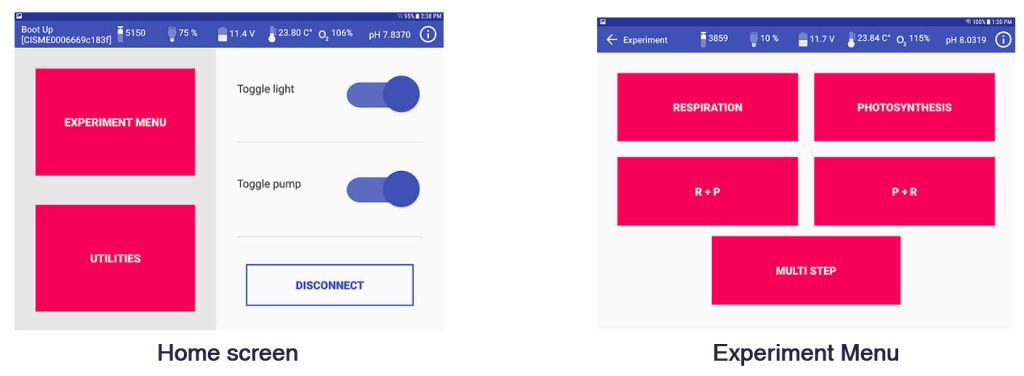
- CISME is operated with a proprietary Android app on a Submersible Android Tablet connected to the CISME by a WiFi cable.
- Recirculating incubation volumes are produced by a closed cell foam seal that traps seawater within CISME against low relief surfaces: corals, reef substrates such as turf and coralline algae, settlement plates.
- Non-destructive sampling for massive corals with low rugosity surfaces. Branching corals require sampling.
- Diver-portable respirometer
- Measures O2 and pH changes in a flow through system
- Water flow rate and light levels set by the user
- Automatic calculations of photosynthesis and respiration
- Uses Android App and submersible tablet
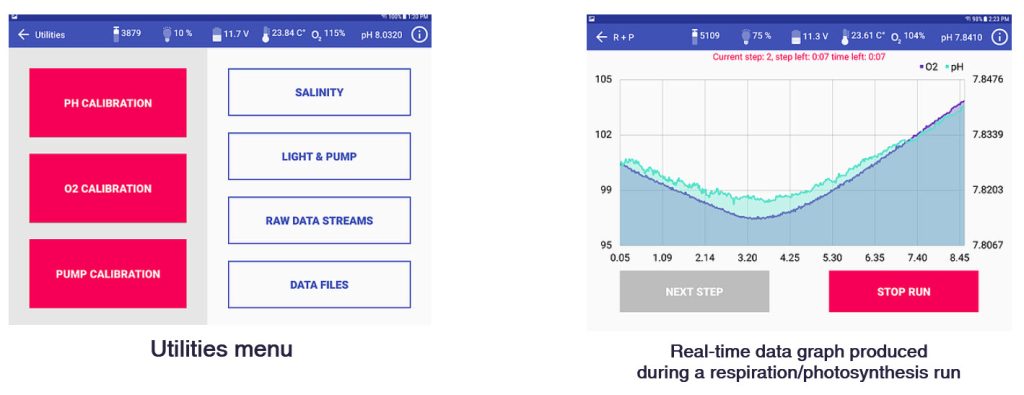
- Measures O2 with Presens® optode and pH with Honeywell DuraFet® electrode at 1 sec intervals.
- Diver Portability: Instrument and underwater tablet together are ca. 5-7 lbs negative UW.
- Programmable flow from 350 to 1200 mL/min; rpm feedback from pump
- Programmable Light levels (PAR): 0 – 2500 µmol photons/m/s
- Programmable incubation routines (R, P, R+P, P+R, Custom multistep); P vs I curves can be pre-programmed
- Removable sample loop volume used to collect incubation water sub-samples or introduce additives
- Incubation volume: 71 mL with no sample loop; 88 mL with a 16 mL sample loop; 120 mL with flow cup
- Optional sample cup for use with coral fragments, algae, small invertebrates and other detached organisms. Core tube adaptor for use in situ
- Designed to maximize data collection by divers: short incubations of 5 to 60 minutes duration, depending on organism and desired measurements. Calcification takes longer than respiration and photosynthesis.
- High capacity external battery power allows up to 8 hours of use before recharging.
- An underwater Android tablets is required for operation. Two options are available.
- Neely KL, Whitehead RF, Dobler MA (2024) The Effects of Disease Lesions and amoxicillin treatment on the physiology of SCTLD-affected corals. Frontiers of Marine Science, Vol11 https://doi.org/10.3389/fmars.2024.1460163
- Dellisanti W. et al. 2023 Seasonal drivers of productivity and calcification in the coral Platygyra carnosa in a subtropical reef. Front. Mar. Sci. https://doi.org/10.3389/fmars.2023.994591
- Romano de Orte M. et al. 2021 Unexpected role of communities colonizing dead coral substrate in the calcification of coral reefs. Limnology and Oceanography March 17, 2021 https://doi.org/10.1002/lno.11722
- Dellisanti et al. 2020. A Diver-Portable Respirometry System for in-situ Short-Term Measurements of Coral Metabolic Health and Rates of Calcification. Front. Mar. Sci., 12 November 2020 https://doi.org/10.3389/fmars.2020.571451
- Dellisanti W., J. Wu, L. Chan. 2018. “Advances in coral in-situ metabolism of Hong Kong coral communities”. 4th Asia-Pacific Coral Reef Symposium (Cebu, Philippines, 4-8 June 2018). Oral Presentation
- Dellisanti W., J. Wu, L. Chan. 2019. “The coral communities in Hong Kong living at the metabolic tolerance limits”. The 12th Marine Science Symposium (Taipei, Taiwan, 14-17 May, 2019). Oral Presentation
- Dellisanti W., T.H. Chung, R.H.L. Tsang, P. Ang, M. L. Wells, J. Wu, L. Chan. 2019. “The coral health status of Platygyra spp. at thermal and salinity tolerance limits in Hong Kong waters”. 9th International Conference on Marine Pollution and Ecotoxicology (Hong Kong, S.A.R. China, 10-14 June 2019). Oral Presentation
- Dellisanti W., R.H.L. Tsang, P. Ang, M. L. Wells, J. Wu, L. Chan. 2019. “The physiological response to high temperature and low salinity of Hong Kong’s Platygyra spp. corals by underwater respirometry observations” The First Graduate Symposium on Marine Environmental Research GRAMMER (Hong Kong, S.A.R. China, 12 March 2019). Oral Presentation
- Dellisanti W., R.H.L. Tsang, P. Ang, J. Wu, L. Chan. 2019. “The short-term plasticity of Hong Kong’s Platygyra spp. corals: a new insight on coral in-situ metabolism and ex-situ manipulations”. The Fourth Xiamen Symposium on Marine Environmental Sciences XMASIV (Xiamen, China, 6-9 January 2019). Oral Presentation
- Dellisanti W., J. Wu, L. Chan. 2019. “The coral communities in Hong Kong living at the metabolic tolerance limits”. The 12th Marine Science Symposium (Taipei, Taiwan, 14-17 May, 2019). Oral Presentation
- Dellisanti W., T.H. Chung, R.H.L. Tsang, P. Ang, M. L. Wells, J. Wu, L. Chan. 2019. “The coral health status of Platygyra spp. at thermal and salinity tolerance limits in Hong Kong waters”. 9th International Conference on Marine Pollution and Ecotoxicology (Hong Kong, S.A.R. China, 10-14 June 2019). Oral Presentation
- Dellisanti W., R.H.L. Tsang, P. Ang, M. L. Wells, J. Wu, L. Chan. 2019. “The physiological response to high temperature and low salinity of Hong Kong’s Platygyra spp. corals by underwater respirometry observations” The First Graduate Symposium on Marine Environmental Research GRAMMER (Hong Kong, S.A.R. China, 12 March 2019). Oral Presentation
- González Casañas F., Szmant A., Whitehead R. and Weil E. 2019. Assessing metabolic changes of the Caribbean coral Orbicella faveolata during its gametogenesis using the CISME (Coral In Situ Metabolism) device. ASLO 2019 Aquatic Sciences Meeting: Planet Water Challenges And Successes. Program book page: 85. Oral presentation.
- Dellisanti W., R.H.L. Tsang, P. Ang, J. Wu, L. Chan. 2019. “The short-term plasticity of Hong Kong’s Platygyra spp. corals: a new insight on coral in-situ metabolism and ex-situ manipulations”. The Fourth Xiamen Symposium on Marine Environmental Sciences XMASIV (Xiamen, China, 6-9 January 2019). Oral Presentation
- González Casañas F., Szmant A., Whitehead R. and Weil E. 2019. Assessing metabolic changes of the Caribbean coral Orbicella faveolata during its gametogenesis using the CISME (Coral In Situ Metabolism) device. ASLO 2019 Aquatic Sciences Meeting: Planet Water Challenges And Successes. Program book page: 85. Oral presentation.
- Romano de Orte, M. 2019. In-situ metabolic measurements reveal complex recovery on a bleached reef. OceanVisions 2019 – Climate Summit “Successes in resilience, adaptation, mitigation, and sustainability. Georgia Tech., April 2019. Program Book pg. 67
What Does CISME Measure?
Screenshots of the Android app