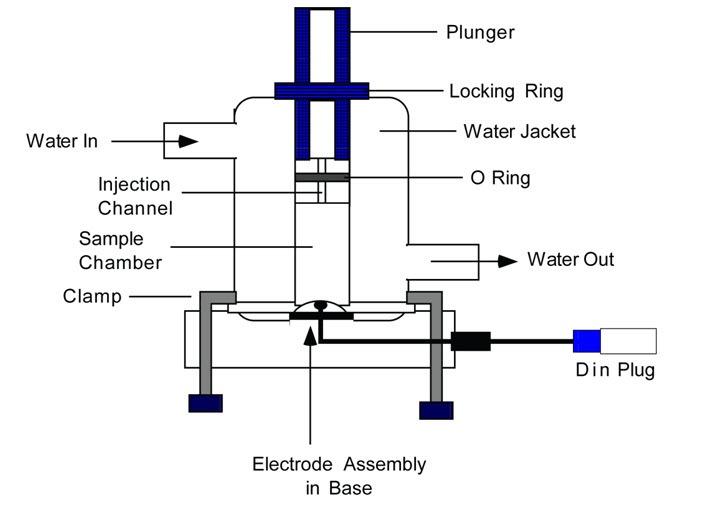
Dissolved Oxygen (DO) Cuvette Electrodes are polarographic oxygen sensing systems for measurements of dissolved oxygen production or consumption in liquid suspension. DO Cuvette Electrode is composed of a water jacketed cuvette, a removable base that houses the electrode, and a plunger. DO cuvette electrodes are available in various volumes 1, 2.5, 4, 6, 30, and 50 ml. A flow-through cuvette is also available in 100 ul volume.
DO cuvette electrodes are based on a Clark cell type detector of dissolved oxygen and can resolve changes of 0.03% O2 in a background of 100% air-saturated water with a fast response time (15s). The DO electrodes are used together with A231 Electrode Amplifier, A255 Magnetic Stirrer, C410 Data interface, and C901 software. These electrodes are integral components of the various OX1LP Dissolved Oxygen Packages. The schematic diagram of the DO cuvette electrode is shown below.
- algal photosynthesis and respiration
- bacterial respiration
- respiration of microorganisms
- respiration of small aquatic animals
- mitochondrial respiration
- chloroplast O2 production
- cell suspension respiration
- plant cells suspension photosynthesis
- Koblizek M et al (2020) Utilization of Light energy in phototropic Gemmatimonadetes. Journal of Photochemistyr and Photobiology B 213: 112085 https://doi.org/10.1016/j.jphotobiol.2020.112085
- Nair P, Huertas M, Nowlin WH (2020) Metabolic responses to long-term food deprivation in subterranean and surface amphipods. Subterranean Biology 33: 1-15 https://digital.library.txstate.edu/handle/10877/9805
- Henry EF, MacCormack TJ (2018) Taurine protects cardiac contractility in killifish, Fundulus heteroclitus, by enhancing sarcoplasmic reticular Ca2+ cycling. Journal of Comparative Physiology B 188:89-99 https://link.springer.com/article/10.1007/s00360-017-1107-4
- Lamarre SG et al. (2019) Interrelationship Between Contractility, Protein Synthesis and Metabolism in Mantle of Juvenile Cuttlefish (Sepia officinalis). Frontiers in Physiology V10, 1-14 (doi: 10.3389/fphys.2019.01051)
- Sirikhachornkit et al. (2016) Increasing the Triacylglycerol Content in Dunaliella tertiolecta through Isolation of Starch-Deficient Mutants. J. Microbiol. Biotechnol. 26(5), 854–866
- Croston, Tara L., et al. (2014) Functional deficiencies of subsarcolemmal mitochondria in the type 2 diabetic human heart. American Journal of Physiology-Heart and Circulatory Physiology 307.1 H54-H65
- Sussarellu, Rossana, et al. (2013) Rapid mitochondrial adjustments in response to short-term hypoxia and re-oxygenation in the Pacific oyster, Crassostrea gigas.” The Journal of Experimental Biology 216.9 1561-1569
- Dickinson GH et al. (2013) Environmental salinity modulates the effects of elevated CO2 levels on juvenile hard-shell clams, Mercenaria mercenaria. The Journal of experimental biology 216.14 2607-2618.
- Bauer I and Kappler A (2009) Rates and Extent of Reduction of Fe(III) Compounds and O2 by Humic Substances. Environ. Sci. Technol. Vol 43, Number 13, p4902–4908
- Mosher CM et al. (2008) Functional Analysis of Phenylalanine Residues in the Active Site of Cytochrome P450 2C9. Biochemistry Vol 47, Number 45, p11725–11734
- Mendelsohn BA et al. (2008).The zebrafish embryo as a dynamic model of anoxia tolerance. Developmental Dynamics Vol 237, Issue 7, p1780–1788
- Mendelsohn BA and Gitlin JD (2008) Coordination of development and metabolism in the pre-midblastula transition zebrafish embryo. Developmental Dynamics Vol 237, Issue 7, p1789–1798.
- Johnson EA, Rosenberg J, McCarty RE. (2007) Expression by Chlamydomonas reinhardtii of a chloroplast ATP synthease with polyhistidine-tagged beta subunits. Biochimica et Biophysica Acta 1767:374-380
- Johnson E A. (2008) Altered expression of the chloroplasts ATP synthase through site-directed mutagenesis inChlamydamonas Reinhardtii. Photosynth Res vol 96:153-162
- Locuson CW, Gannett PM and Tracy TS (2006) Heteroactivator effects on the coupling and spin state equilibrium of CYP2C9. Archives of Biochemistry and Biophysics Vol 449, Issues 1-2, p115-129
The following DO cuvette Electrodes are available, the maximum volume of the electrode is indicated. The minimum volume is 1/6th of the maximum and it can be achieved by lowering the plunger into the sample chamber:
- 1ml perspex (G108)
- 2.5ml perspex (G105)
- 2.5ml glass (G105-GL)
- 4ml perspex (G110)
- 6ml glass (G106-GL)
- 30ml perspex (G109)
- 50ml perspex (G107)
- 50ml glass (G107-GL)
- 100ul flow through (G90-FL)