Z985 Cuvette AquaPen is a version of the Z980 AquaPen chlorophyll fluorometer. It has blue and red measuring lights that enable chlorophyll fluorescence measurements of photosynthetic parameters both in algal and cyanobacterial suspensions in a cuvette. Due to its ultra-high sensitivity – up to 0.5 μg Chl/L, the Cuvette AquaPen can make measurements in natural water samples containing low concentrations of phytoplankton.
The Z985 Cuvette AquaPen includes the chlorophyll fluorescence kinetics protocols for measurements of OJIP, NPQ, and Light Curve responses of QY. In addition, optical density (OD) at 680nm and 720nm can also be measured. The unit has both data transfer options via Bluetooth and USB communication technology. With a built-in GPS module and a rechargeable Li-ion battery, it is ideal for use in the field. The AquaPen comes with PC software for data transfer and visualization. A Probe version of the AquaPen (Z980) allows the same measurements of chlorophyll fluorescence in suspension by directly placing the probe in the suspension medium.
- Hand-held chlorophyll fluorometer for algal suspensions placed in a cuvette
- High chlorophyll detection sensitivity – down to 0.5 ug chl/L
- Includes 4ml cuvettes
- Measurements of Optical Density at 680 and 720nm
- Software and data transfer to PC module via USB and Bluetooth included
- GPS module included
- Rechargeable Li-ion battery
- Photosynthesis research of algal and cyanobacterial suspensions
- Photosynthesis education
- Phycology
- Limnology
- Oceanography
- Biotechnology
- Phenotyping/screening of algal strains
- Measured/Calculated Parameters:F0 , FT , FM , FM ‘ , QY, OJIP, NPQ1, NPQ2, NOQ3, LC 1, LC 2, LC3, OD 680 , OD 720
- Actinic and Saturating Light: Adjustable from 0 to 1000 and 3,000 µmol (photons)/m2/s1, respectively
- Measuring Light: Red and blue measuring lights adjustable by intensity
- Detector Wavelength Range: PIN photodiode with 667 to 750 nm bandpass filters
- FluorPen 1.1 Software: for Windows 7, or higher
- Memory Capacity: Up to 16 Mb
- Communication for data transfer: USB and Bluetooth
- Internal Data Logging: Up to 149,000 data points
- Display: 2 x 8 characters LC display
- Keypad: Sealed, 2-key tactile response
- Keypad Escape Time: Turns off after 8 minutes of no use
- Power Supply: Li-ion rechargeable battery
- Battery Life: 48 hours typical with full operation
- Low Battery Detection: Low battery indication displayed
- Size: 165 x 65 x 55 mm mm
- Weight: 290 g,
- Sample Holder: 4 ml cuvette
- Operating Conditions: Temperature: 0 to 55ºC; 32 to 130ºF. Relative humidity: 0 to 95 % (non-condensing)
- Storage Conditions: Temperature: -10 to +60ºC; 14 to +140ºF. Relative humidity: 0 to 95 % (non-condensing)
- Warranty: 1 year parts and labor
- GOIRIS K., VAN COLEN W., WILCHES I. ET AL. (2015): Impact of nutrient stress on antioxidant production in three species of microalgae. Algal Research. Volume 7, Pages 51-57. DOI:10.1016/j.algal.2014.12.002
- DE MARCHIN T., GHYSELS B., NICOLAY S. ET AL. (2014) Analysis of PSII antenna size heterogeneity of Chlamydomonas reinhardtii during state ransitions. Biochimica et Biophysica Acta (BBA) – Bioenergetics, Volume 1837, Pages 121-130. DOI:10.1016/j.bbabio.2013.07.009
- MALAPASCUA J. R. F., JEREZ C. G., SERGEJEVOVÁ M. ET AL. (2014). Photosynthesis monitoring to optimize growth of microalgal mass cultures: application of chlorophyll fluorescence techniques. Aquatic Biology; Volume 22, Pages 123–140. DOI: 10.3354/ab00597
- SALEH M. M., MATORIN D. N., ZAYADAN B. K. ET AL. (2014). Differentiation between two strains of microalga Parachlorella kessleriusing modern spectroscopic method. Botanical Studies, Pages 55-53. DOI: 10.1186/s40529-014-0053-7
- THRANE J. E., HESSEN D. O. AND ANDERSEN T. (2014). The Absorption of Light in Lakes: Negative Impact of Dissolved Organic Carbon on Primary Productivity; Ecosystems. Volume 17: Pages 1040–1052. DOI: 10.1007/s10021-014-9776-2
- THOMMER G., LEYNAERT A., KLEIN C. ET AL. (2013). Phytoplankton phosphorus limitation in a North Atlantic coastal ecosystem not predicted by nutrient load. Journal of Plankton Research. 0(0). Pages 1 – 13. DOI:10.1093/plankt/fbt070
- LAZÁR D, MURCH S. J., BEILBY M. J. ET AL. (2013). Exogenous melatonin affects photosynthesis in characeae Chara australis; Plant Signaling and Behavior. Volume 8(3): e23279. DOI: 10.4161/psb.23279
- CAMERON J. C., WILSON S. C., BERNSTEIN S. L. ET AL. (2013). Biogenesis of a Bacterial Organelle: The Carboxysome Assembly Pathway;; Cell, Volume 155, Issue 5, Pages 1131-1140. DOI:10.1016/j.cell.2013.10.044
- FIGUEROA F. L., JEREZ C. G. AND KORBEE N. (2013). Use of in vivo chlorophyll fluorescence to estimate photosynthetic activity and biomass productivity in microalgae grown in different culture systems. Latin American Journal of Aquatic Research. Volume 41, no. 5, Pages 801-819. DOI: 103856/vol41-issue5-fulltext-1
- OSANAI T., KUWAHARA A., IIJIMA H.ET AL. (2013). Pleiotropic effect of sigE over-expression on cell morphology, photosynthesis and hydrogen production in Synechocystis sp. PCC 6803; The Plant Journal. Volume 76, Pages 456–465. DOI: 10.1111/tpj.12310
- ZAKERI H. A. AND BAKAR L. A. (2013). Copper-, Lead- and Mercury-Induced Changes in Maximum Quantum Yield, Chlorophyll A Content and Relative Growth of Three Malaysian Green Macroalgae; Malaysian Journal of Fundamental and Applied Sciences. Voume 9, Pages 16-21.
- ALAMI M, LAZAR D AND GREEN B. R. (2012). The harmful alga AUREOCOCCUS ANOPHAGEFFERENS utilizes 19′-butanoyloxyfucoxanthin as well as xanthophyll cycle carotenoids in acclimating to higher light intensities;; Biochimica et Biophysica Acta (BBA) – Bioenergetics; Volume 1817, Issue 9, Pages 1557–1564 DOI:10.1016/j.bbabio.2012.05.006
- LELONG A., HÉGARET H. AND SOUDANT P. (2011). Cell-based measurements to assess physiological status of Pseudo-nitzschia multiseries, a toxic diatom; Research in Microbiology, Volume 162, Issue 9, Pages 969-98. DOI:10.1016/j.resmic.2011.06.005
- STALEY Z. R., ROHR J. R. AND HARWOOD V. J.( 2011). Test of Direct and Indirect Effects of Agrochemicals on the Survival of Fecal Indicator Bacteria. Applied and Environmental Microbiology. Pages 8765–8774. DOI: 10.1128/AEM.06044-11
- Q.T. GAO AND N.F.Y. TAM. (2011). Growth, photosynthesis and antioxidant responses of two microalgal species, CHLORELLA VULGARIS and SELENASTRUM CAPRICORNUTUM, to nonylphenol stress. Chemosphere; Volume 82, Issue 3, Pages 346–354. DOI:10.1016/j.chemosphere.2010.10.010
- VANDAMME D, FOUBERT I., MEESSCHAERT B.ET AL (2010). Flocculation of microalgae using cationic starch; Journal of Applied Phycology, Volume 22, Issue 4, Pages 525-530. DOI: 10.1007/s10811-009-9488-8
- KVÍDEROVÁ JANA. (2010). Rapid algal toxicity assay using variable chlorophyll fluorescence for Chlorella kessleri (chlorophyta). Journal of Environmental Toxicology. Volume 25(6), Pages 554-63. DOI:10.1002/tox.20516
- KROMKAMP J. C., BEARDALL J., SUKENIK A. ET AL. (2009). Short-term variations in photosynthetic parameters of Nannochloropsis cultures grown in two types of outdoor mass cultivation systems. Aquatic Microbial Ecology; Volume 56, Pages 309–322. DOI: 10.3354/ame01318
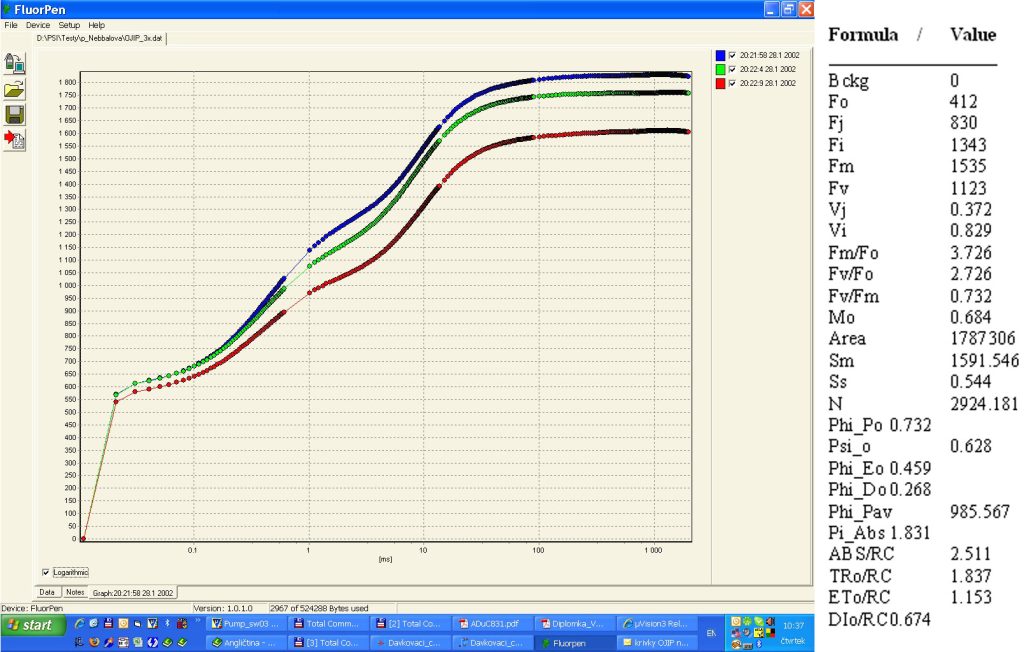
- Bckg = background
- F0: = F50µs; fluorescence intensity at 50 µs
- FJ: = fluorescence intensity at j-step (at 2 ms)
- Fi: = fluorescence intensity at i-step (at 60 ms)
- FM: = maximal fluorescence intensity
- FV: = FM – F0 (maximal variable fluorescence)
- VJ = (FJ – F0) / (FM – F0)
- Vi = (Fi – F0) / (FM – F0)
- FM / F0
- FV / F0
- FV/ FM
- M0 or (dV / dt)0 = TR0 / RC – ET0 / RC = 4 (F300 – F0) / (FM – F0)
- Area = area between fluorescence curve and FM (background subtracted)
- Fix Area = total area above the OJIP fluorescence transient – between F40µ and F1s(background subtracted)
- SM = area / FM – F0 (multiple turn-over)
- Ss = the smallest Sm turn-over (single turn-over)
- N = SM . M0 . (1 / VJ) turn-over number QA
- Phi_P0 = 1 – (F0 / FM (or FV / FM)
- Psi_0 = 1 – VJ
- Phi_E0 = (1 – F0 / FM)) . Psi_0
- Phi_D0 = 1 – Phi_P0 – (F0 / FM)
- Phi_Pav = Phi_P0 – (SM / tFM); tFM) = Time to reach FM (in ms)
- ABS / RC = M0 . (1 / VJ) . (1 / Phi_P0)
- TR0 / RC = M0 . (1 / VJ)
- ET0 / RC = M0 . (1 / VJ) . Phi_0)
- DI0 / RC = (ABS / RC) – (TR0 / RC)
Formulas Derived From:
R.J. Strasser, A. Srivastava and M. Tsimilli-Michael (2000): The fluorescence transient as a tool to characterize and screen photosynthetic samples. In: Probing Photosynthesis: Mechanism, Regulation and Adaptation (M. Yunus, U. Pathre and P. Mohanty, eds.), Taylor and Francis, UK, Chapter 25, pp 445-483.
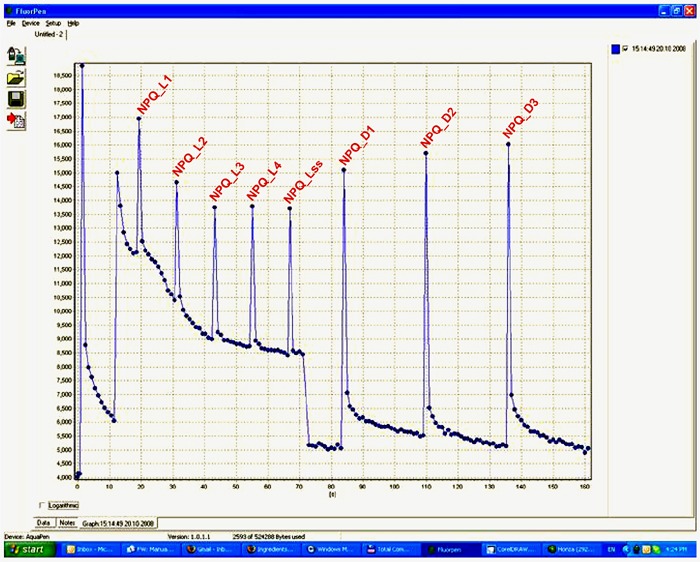
NPQ and Light Curve Protocols
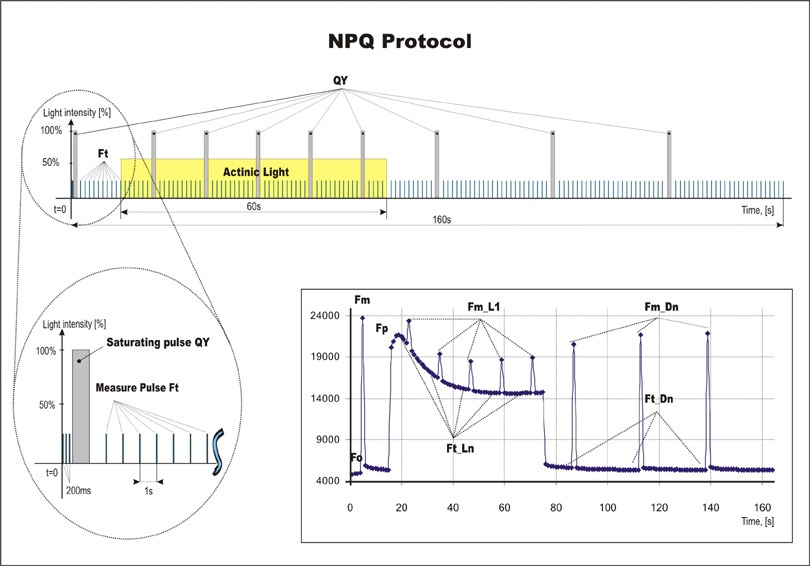
- Three predefined NPQ protocols differing in the duration of light exposure and dark recovery phase as well as in the number of intervals between the pulses
- Typically used for quantification of photochemical and non-photochemical quenching in dark-adapted samples
- NPQ 1 protocol: light duration 60s, 5 pulses; dark recovery duration 88s, 3 pulses
- NPQ 2 protocol: light duration 200s, 10 pulses; dark recovery duration 390s, 7 pulses
- NPQ3 protocol:light duration 200s, 10 pulses; dark recovery duration 60s, 2 pulses
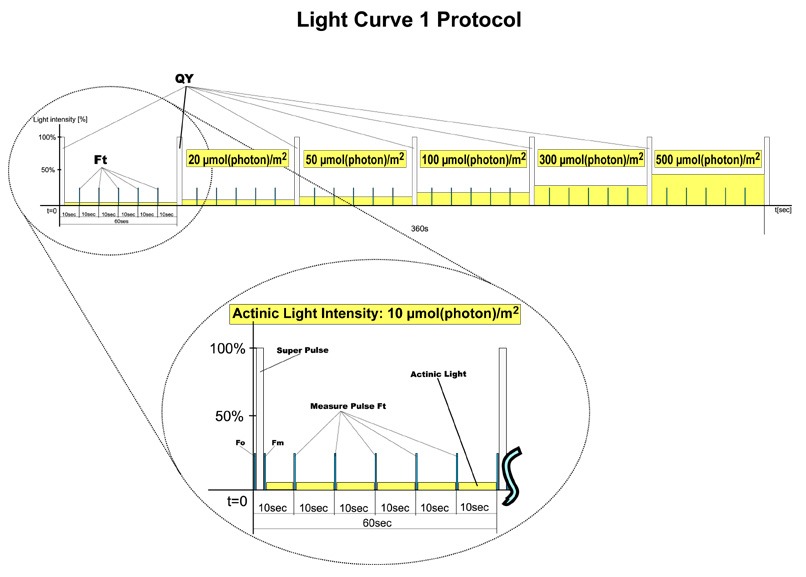
- Three predefined protocols differing in number and duration of light phases and light intensities
- Based on pulse modulated fluorometry (PAM)
- The effective quantum yields of photosynthesis are determined under various light intensities of continuous illumination.
- Light response curve relating the rate of photosynthesis to photon flux density
- LC1 protocol: 6 phases with 60s duration (10, 20, 50, 100, 300, 500 uE)
- LC2 protocol: 5 phases with 30s duration (100, 200, 300, 500, 1000uE)
- LC3 protocol: 7 phases with 60s duration (10, 20, 50, 100, 300, 500, 1000uE)