
The Z300 Handy FluorCam is designed for kinetically resolved chlorophyll fluorescence imaging of leaves, small plants, stems, seeds, roots, and tissues on plates both in a field and laboratory. The imaging area is up to 3.5 x 3.5 cm. The essential features of the Z300 Handy FluorCam are similar to the Z100 FluorCam Closed.
The Z300 Handy FluorCam is a portable system and can be used in the field. Optional accessories include sealed lead acid batteries in a convenient bag carried on the shoulder, an ultra-light tripod, and a leaf clip.
Z300 Handy FluorCam generates images of fluorescence signal and presents them using a false color scale. Full kinetic analysis is available.
In all applications, the camera allows imaging of fluorescence transients that are induced by actinic light or by saturating flashes. The timing and amplitude of actinic irradiance are determined by user-defined protocols. The FluorCam software package (included along with a PC computer) provides a Wizard with the most frequently used experimental protocols. For an experienced professional, the software offers a sophisticated programming language that can be used for designing novel timing and measuring sequences.
- High resolution CCD camera based imaging system
- 4 LED panels for standard excitation
- User friendly software with protocols for Fv/Fm, Kautsky induction, Quenching analysis, Light Curve, steady state fluorescence (standard)
- Measurements of up to 50 different chlorophyll fluorescence paramters
- Imaging of other fluorescent proteins (GFP, YFP etc) with optional filters and LED panel
- Small and portable system for both laboratory and field use
- Screening for photosynthetic performance via chlorophyll fluorescence.
- Investigations of heterogeneity within a single leaf, e.g., for infection, necrotic changes, senescence, abiotic stress.
- Biotic and abiotic stress resistance in plants
- Plant pathogen research
- Stomatal patchiness studies
- Growth and Yield Improvements in Plants
- Fluorescence Parameters:
– Measured parameters: FO, FM, FV, FO’, FM’, FV’, FT
– More than 50 calculated parameters: FV/FM, FV’/FM’, PhiPSII , NPQ, qN, qP, Rfd, PAR-absorptivity coefficient, and many others - Excitation Light Sources:
– Measuring pulses: orange (620 nm)
– Actinic / saturating light: white (standard) or blue (455 nm) or orange (620 nm) - Saturating Pulse Intensity:
white: up to 3,900 µmol(photons)/m²/s
blue: up to 4,900 µmol(photons)/m²/s
red-orange: up to 3,800 µmol(photons)/m²/s - Actinic Light Intensity::
white: approx. 1,000 µmol(photons)/m²/s
blue: approx. 1,400 µmol(photons)/m²/s
red-orange: approx. 1,000 µmol(photons)/m²/s - Light Regime:
Static or sinus form (hub connected) - Custom-Defined Protocols:
Variable timing, special language and scripts - CCD Detector Wavelength Range:
400 – 1000 nm - CCD Format:
720 x 560 pixels; optionally 1024 x 768 pixels (video mode) or 1392 x 1040 pixels (snapshot mode) - Pixel Size:
8.2 µm x 8.4 µm; optionally 6.45 µm x 6.45 µm - A/D Bit Resolution:
12 bit - Spectral Response:
QE max at 540 nm (~70 %), 50 % roll-off at 400 nm and 650 nm - Read-out Noise:
Less than 12 electrons RMS – typically only 10 electrons - Full-Well Capacity:
Greater than 70,000 electrons (unbinned) - Imaging Frequency:
Maximum 50 frames per second - Bios:
Upgradeable firmware - Communication Port:
USB 2.0 - Handy FluorCam Weight:
1.8 kg - Leaf-Clip Weight:
0.2 kg - Power Supply Weight:
2.5 kg - FluorCam Stand Weight:
1.5 kg - Notebook Weight (incl. all accessories):
3.5 kg - Power Input:
Max. 200 W - Electrical:
90 – 260 V
- MASCALCHI M., OSTICIOLI I., RIMINESI C. et al. (2015): Preliminary investigation of combined laser and microwave treatment for stone biodeterioration. Studies in Conservation. Volume 60, Pages 19-27. DOI: 10.1179/0039363015Z.000000000203
- KWON M. Y., KIM S. H., SUNG J. H. (2014): The responses of Growth and Physiological traits of Acer triflorum on Calcium Chloride (CaCl2) Concentration1 Korean Journal of Environment and Ecology. Volume 28, Pages 500-509 DOI: 10.13047/KJEE.2014.28.5.500
- LI X., ZHAO W., SUN X. et al. (2013): Molecular cloning and characterization of violaxanthin de-epoxidase (CsVDE) in cucumber. PLoS One. 8(5):e64383. DOI: 10.1371/journal.pone.0064383
- VREDENBERG W. and PAVLOVIC A. (2013): Chlorophyll a fluorescence induction (Kautsky curve) in a Venus flytrap (Dionaea muscipula) leaf after mechanical trigger hair irritation. J. Plant Physiol. 170(3): 242-50. DOI: 10.1016/j.jplph.2012.09.009
- ELLAWALA C., ASAEDA T., and KAWAMURA K. (2011): Influence of flow turbulence on Chara fibrosa: growth, stress, and tissue carbon content. J. Freshwater Ecology 26(4): 507–515. DOI: 10.1080/02705060.2011.595555
- FERNÁNDEZ-MARÍN B., MÍGUEZ F., BECERRIL J. M. et al. (2011): Activation of violaxanthin cycle in darkness is a common response to different abiotic stresses: a case study in Pelvetia canaliculata. BMC Plant Biology. Volume 11. DOI:10.1186/1471-2229-11-181
- GABILLY S. T., KROPAT J., KARAMOKO M., ET AL.(2011): A Novel Component of the Disulfide-Reducing Pathway Required for Cytochrome c Assembly in Plastids. Genetics. Volume 187, Pages 793–802. DOI: 10.1534/genetics.110.125369
- MISHRA A., MISHRA K.B., HOERMILLER I.I. et al. (2011): Chlorophyll fluorescence emission as a reporter on cold tolerance in Arabidopsis thaliana accessions. Plant Signaling & Behavior 6(2):301-310. DOI:10.4161/psb.6.2.15278
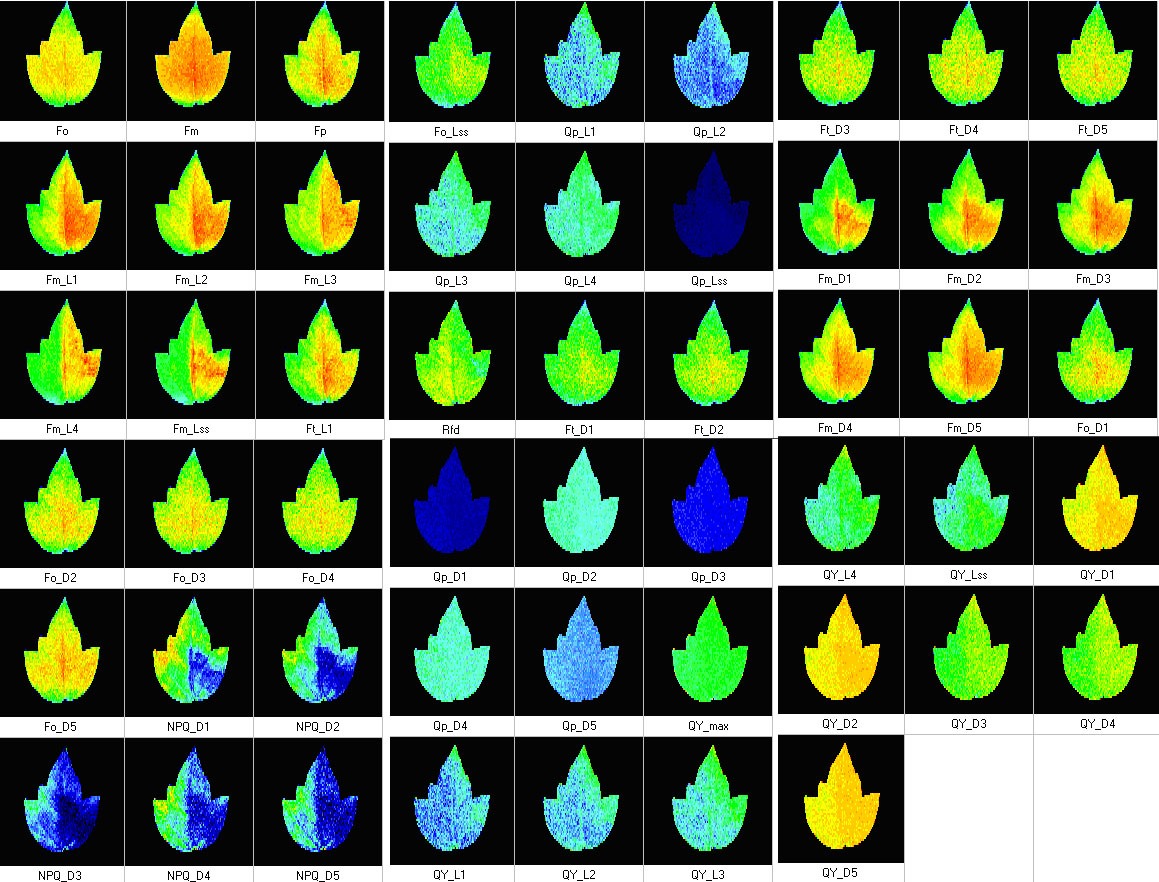
Measured Parameters:
- Quenching.
- Kautsky induction.
- QA reoxidation (needs optional accessories).
- Measured parameters: Fo, FM, FV, Fo’, FM’, FV’, QY(II).
- More than 50 calculated parameters: NPQ, FV/FM, FV’/FM’, Rfd, qN, qP, PAR-absorptivity, electron transport rate (ETR), and many others.
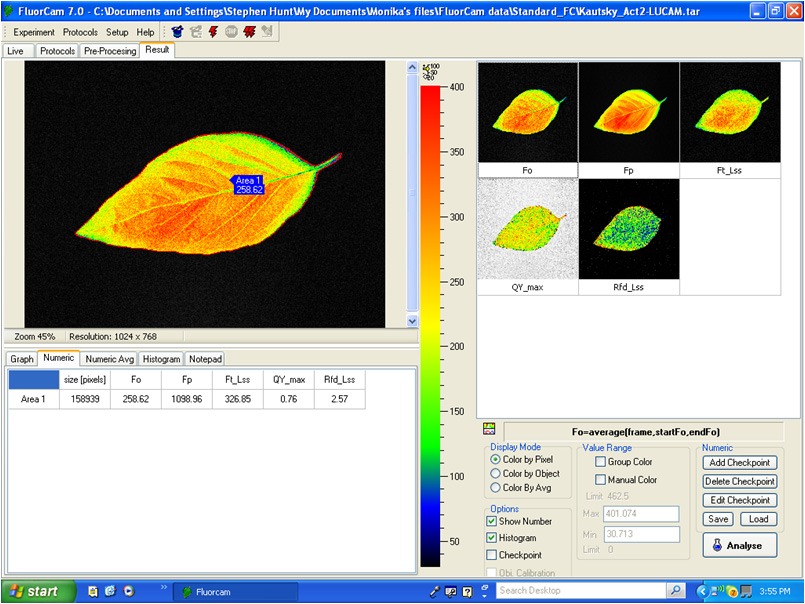
Software Features:
- Automated experimental protocols via a Windows Wizard.
- Multiple (automatically repeated) experiments.
- Automated labeling of individual plants, or samples, within the field of view.
- Kinetic analysis of data from all samples within the field of view.
- Numerous image manipulation tools.
- Barcode reader support (optional)
- Export to Excel.
- Windows 2000, XP, Vista compatible




